Exhibit 99.2

Ocuphire Corporate Presentation May 2023

Disclosures and Forward-Looking Statements This presentation contains
“forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements concerning the success and timing of planned regulatory filings and
approvals, pre-commercial activities, commercialization strategy and timelines, business strategy, product labels, cash runway, scalability, future clinical trials in presbyopia (P), dim light/night vision disturbance (DLD) and diabetic
retinopathy (DR) / diabetic macular edema (DME), including the potential for Nyxol to be a “best in class” presbyopia drop, and timing of planned future clinical trials for APX3330, timing and occurrence of an End-of-Phase 2 meeting with the
FDA, the potential of a Phase 3 registration path for APX3330, the success and timing of planned regulatory filings, business strategy, cash runway, scalability, the potential for APX3330 to be the first line of therapy for DR patients, and
the potential market opportunity for the slowing of DR progression. These forward-looking statements are based upon the Company’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual
results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, including, without limitation: (i) the success, costs, and timing of regulatory
submissions and pre-clinical and clinical trials, including enrollment and data readouts; (ii) regulatory requirements or developments; (iii) changes to clinical trial designs and regulatory pathways; (iv) changes in capital resource
requirements; (v) risks related to the inability of Ocuphire to obtain sufficient additional capital to continue to advance its product candidates and its preclinical programs; (vi) legislative, regulatory, political and economic
developments, (vii) changes in market opportunities, (viii) risks that the partnership with Viatris may not facilitate the commercialization or market acceptance of Ocuphire’s product candidates; (ix) the success and timing of
commercialization of any of Ocuphire’s product candidates, including the scalability of Ocuphire’s product candidates and (x) the maintenance of Ocuphire’s intellectual property rights. The foregoing review of important factors that could
cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors detailed in documents that have been
and may be filed by the Company from time to time with the SEC. All forward-looking statements contained in this presentation speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to
reflect events that occur or circumstances that exist after the date on which they were made. The Company makes no representation or warranty, express or implied, as to the accuracy or completeness of the information contained in or
incorporated by reference into this presentation. Nothing contained in or incorporated by reference into this presentation is, or shall be relied upon as, a promise or representation by the Company as to the past or future. The Company
assumes no responsibility for the accuracy or completeness of any such information. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market shares and other data about our
industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The trademarks included herein are the property of the owners thereof and are used for reference purposes
only. Such use should not be construed as an endorsement of such products. 2
Corporate Highlights Two Lead Clinical-Stage Novel Drugs Addressing Multiple
Large Ophthalmology Markets with Limited to No Competition & Extensive Patent Portfolio Global License Agreement with Viatris to Fund the Development and Commercialization of Nyxol for All Indications Strong Financial Position to
Advance APX 3330 and Nyxol Clinical Programs into 2025 Nyxol for RM Indication PDUFA Date on September 28, 2023 APX3330 – Paradigm Changing Oral Tablet for 8 million DR patients; Moving into Phase 3 APX3330 oral tablets Diabetic
Retinopathy/Diabetic Macular Edema (DR/DME) Nyxol eyedrops Reversal of Mydriasis (RM) – eye dilation Presbyopia (P) – age-related blurry near vision Dim Light or Night Vision Disturbances (DLD) 3
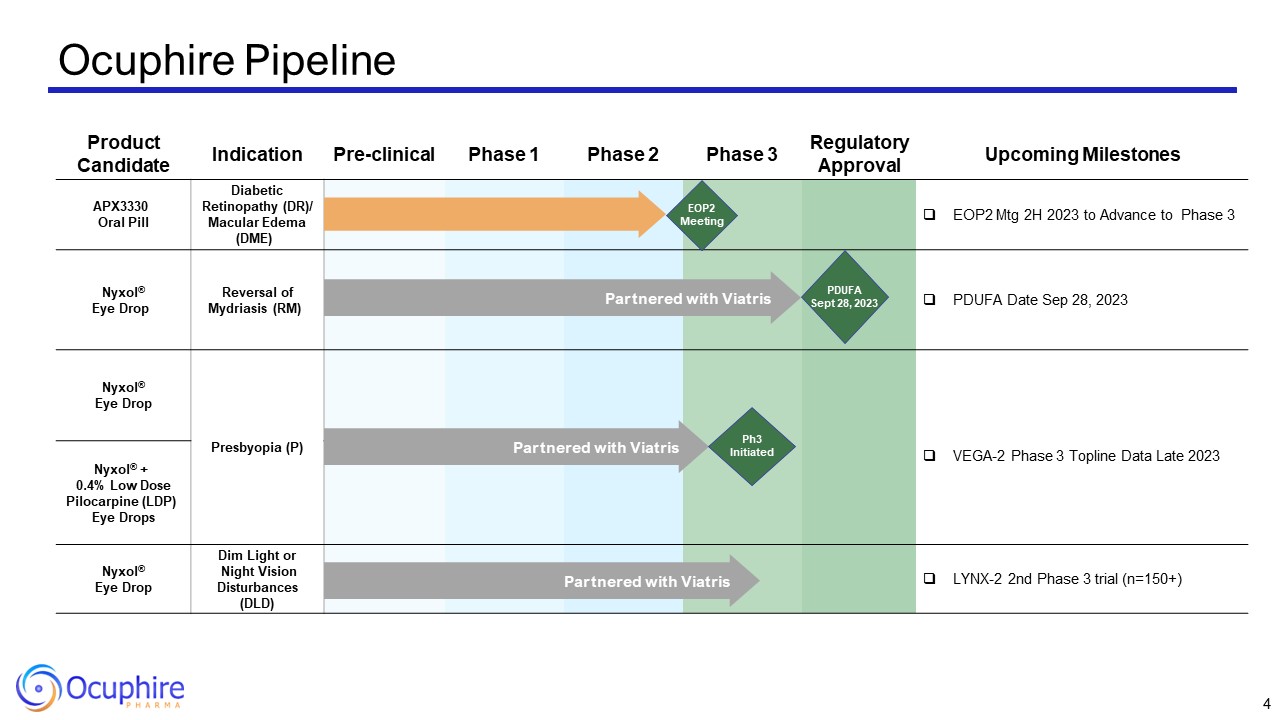
Product Candidate Indication Pre-clinical Phase 1 Phase 2 Phase
3 Regulatory Approval Upcoming Milestones APX3330 Oral Pill Diabetic Retinopathy (DR)/ Macular Edema (DME) EOP2 Mtg 2H 2023 to Advance to Phase 3 Nyxol® Eye Drop Reversal of Mydriasis (RM) PDUFA Date Sep 28, 2023 Nyxol® Eye
Drop Presbyopia (P) VEGA-2 Phase 3 Topline Data Late 2023 Nyxol® + 0.4% Low Dose Pilocarpine (LDP) Eye Drops Nyxol® + 0.4% Low Dose Pilocarpine (LDP) Eye Drops Nyxol® Eye Drop Dim Light or Night Vision Disturbances
(DLD) LYNX-2 2nd Phase 3 trial (n=150+) Ocuphire Pipeline Partnered with Viatris Partnered with Viatris Partnered with Viatris EOP2 Meeting PDUFA Sept 28, 2023 Ph3 Initiated 4
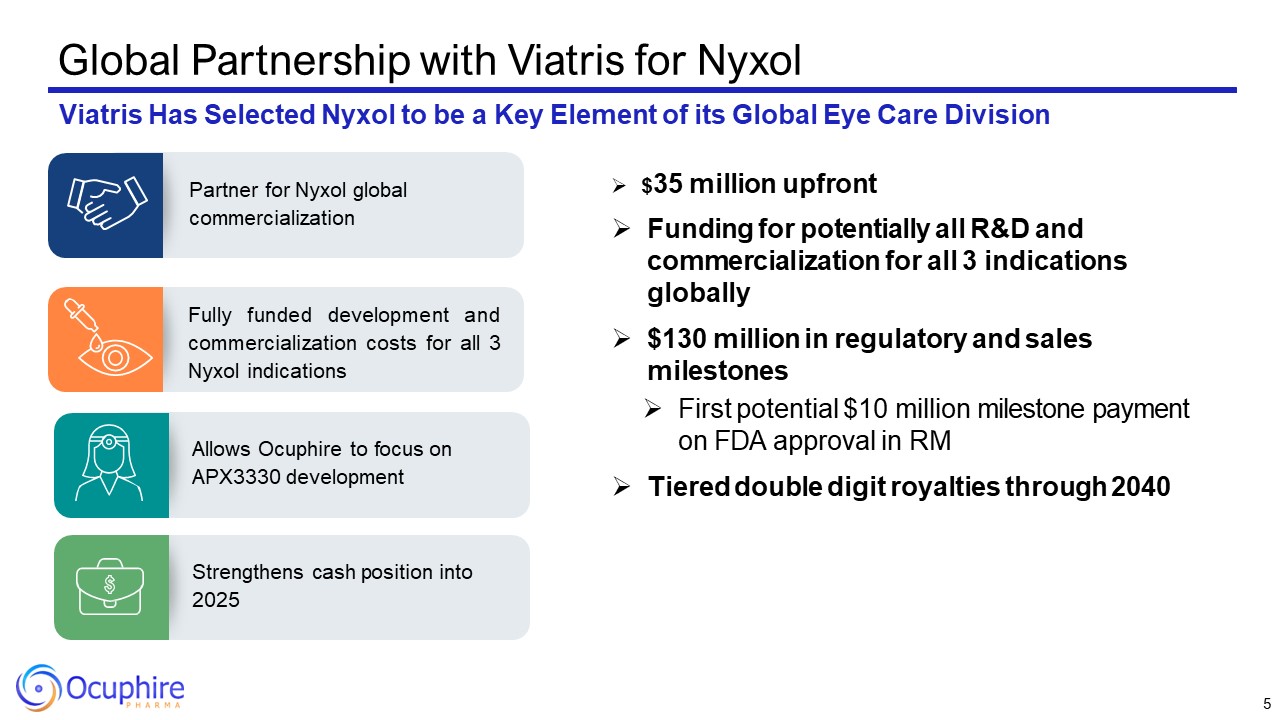
Global Partnership with Viatris for Nyxol Viatris Has Selected Nyxol to be a
Key Element of its Global Eye Care Division Fully funded development and commercialization costs for all 3 Nyxol indications Partner for Nyxol global commercialization Allows Ocuphire to focus on APX3330 development Strengthens cash
position into 2025 $35 million upfront Funding for potentially all R&D and commercialization for all 3 indications globally $130 million in regulatory and sales milestones First potential $10 million milestone payment on FDA approval
in RM Tiered double digit royalties through 2040 5
6 APX3330 ORAL TABLET DR Diabetic Retinopathy DME Diabetic Macular
Edema “ ” “I could lose my hearing, I could lose talking but…. It’s frightening to lose my eyesight.” Patient Diagnosed with DR
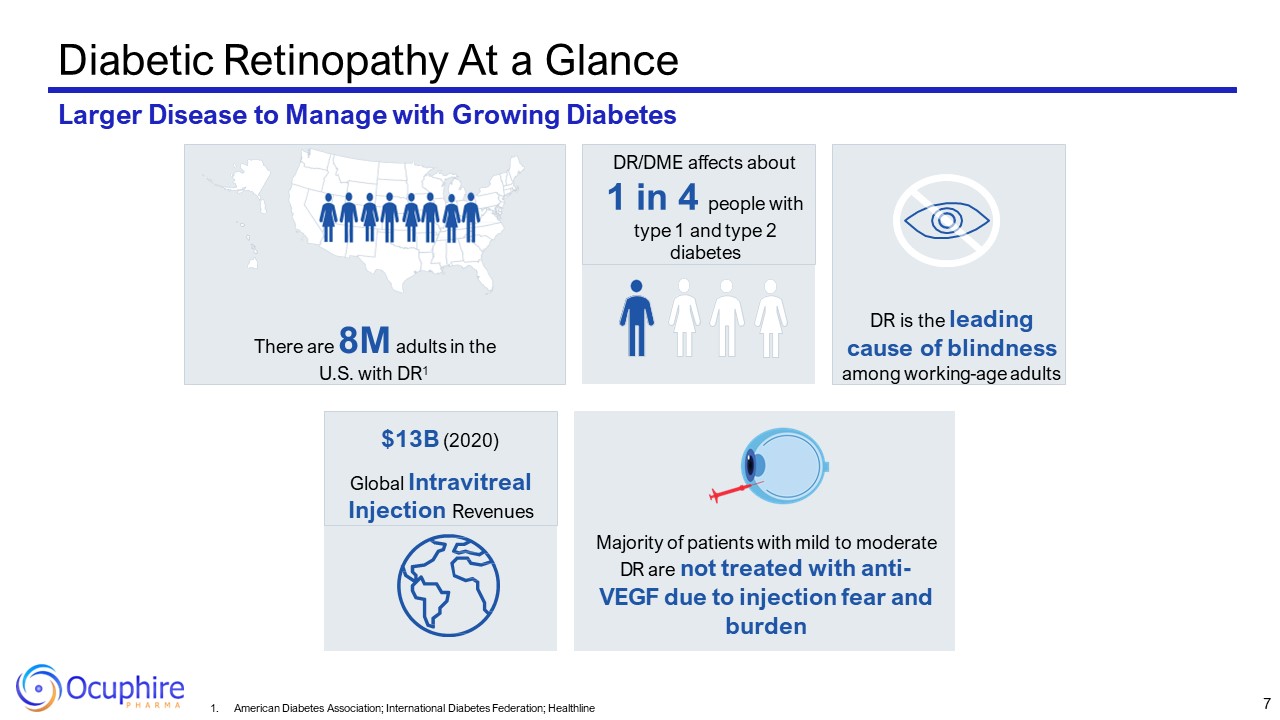
Diabetic Retinopathy At a Glance Larger Disease to Manage with Growing
Diabetes There are 8M adults in the U.S. with DR1 DR is the leading cause of blindness among working-age adults DR/DME affects about 1 in 4 people with type 1 and type 2 diabetes Majority of patients with mild to moderate DR are not
treated with anti- VEGF due to injection fear and burden $13B (2020) Global Intravitreal Injection Revenues 7 American Diabetes Association; International Diabetes Federation; Healthline
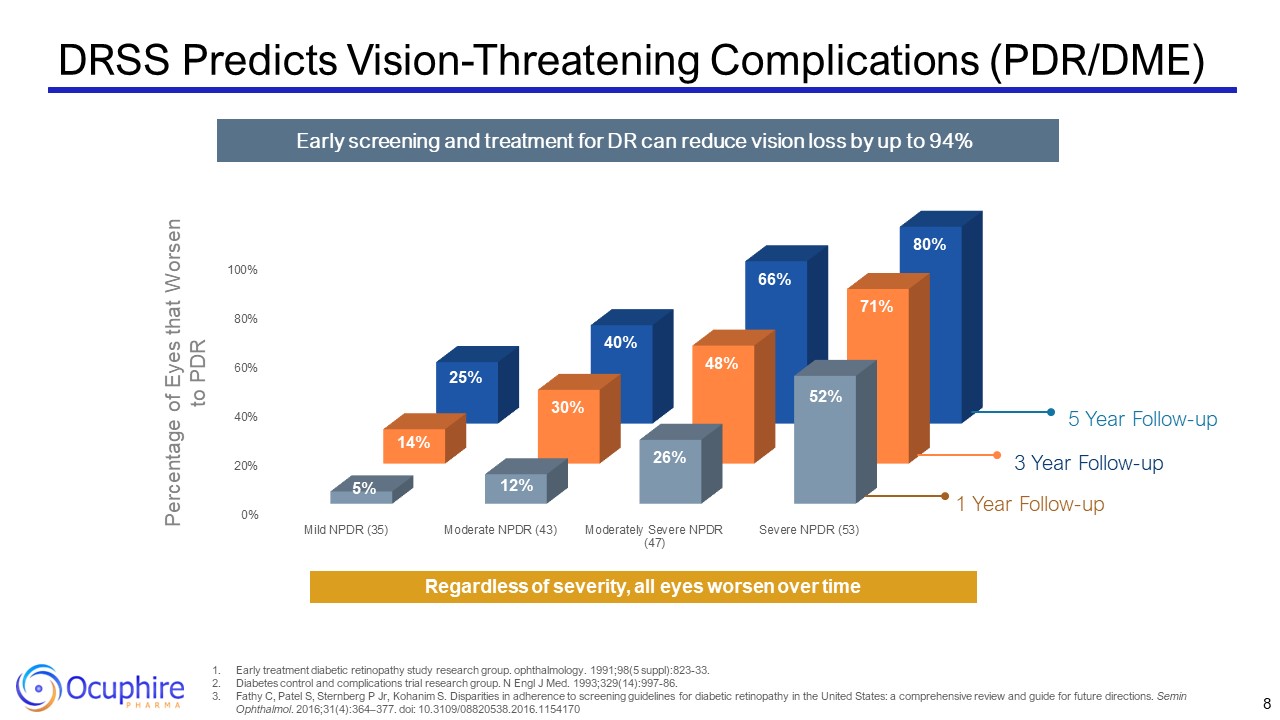
DRSS Predicts Vision-Threatening Complications (PDR/DME) 8 Early treatment
diabetic retinopathy study research group. ophthalmology. 1991;98(5 suppl):823-33. Diabetes control and complications trial research group. N Engl J Med. 1993;329(14):997-86. Fathy C, Patel S, Sternberg P Jr, Kohanim S. Disparities in
adherence to screening guidelines for diabetic retinopathy in the United States: a comprehensive review and guide for future directions. Semin Ophthalmol. 2016;31(4):364–377. doi: 10.3109/08820538.2016.1154170 Early screening and treatment
for DR can reduce vision loss by up to 94% 1 Year Follow-up 3 Year Follow-up 5 Year Follow-up Percentage of Eyes that Worsen to PDR Regardless of severity, all eyes worsen over time
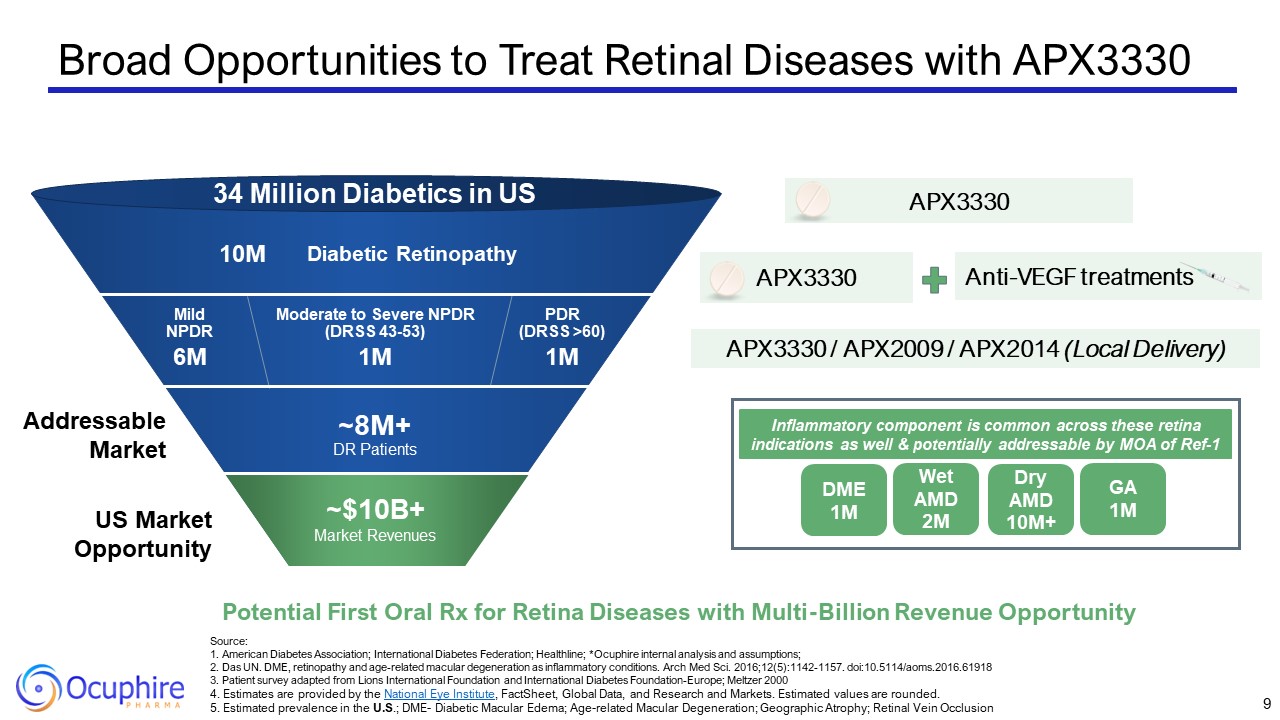
Broad Opportunities to Treat Retinal Diseases with APX3330 Source:1. American
Diabetes Association; International Diabetes Federation; Healthline; *Ocuphire internal analysis and assumptions; 2. Das UN. DME, retinopathy and age-related macular degeneration as inflammatory conditions. Arch Med Sci.
2016;12(5):1142-1157. doi:10.5114/aoms.2016.61918 3. Patient survey adapted from Lions International Foundation and International Diabetes Foundation-Europe; Meltzer 2000 4. Estimates are provided by the National Eye Institute, FactSheet,
Global Data, and Research and Markets. Estimated values are rounded. 5. Estimated prevalence in the U.S.; DME- Diabetic Macular Edema; Age-related Macular Degeneration; Geographic Atrophy; Retinal Vein Occlusion 9 Diabetic
Retinopathy PDR(DRSS >60) Moderate to Severe NPDR (DRSS 43-53) Mild NPDR 34 Million Diabetics in US 10M 6M 1M 1M ~8M+DR Patients Anti-VEGF treatments APX3330 APX3330 / APX2009 / APX2014 (Local Delivery) US Market
Opportunity Addressable Market Potential First Oral Rx for Retina Diseases with Multi-Billion Revenue Opportunity APX3330 ~$10B+ Market Revenues DME 1M Wet AMD 2M Dry AMD 10M+ GA 1M Inflammatory component is common across
these retina indications as well & potentially addressable by MOA of Ref-1
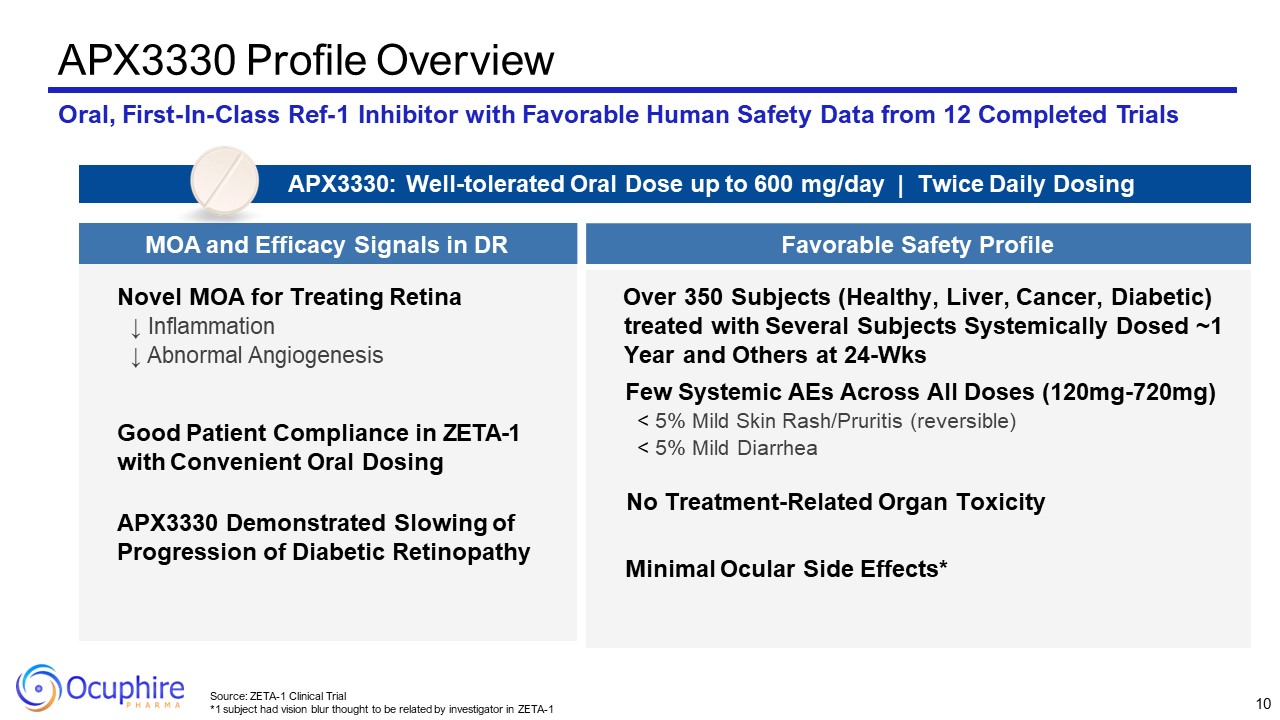
APX3330 Profile Overview Oral, First-In-Class Ref-1 Inhibitor with Favorable
Human Safety Data from 12 Completed Trials Novel MOA for Treating Retina ↓ Inflammation ↓ Abnormal Angiogenesis Good Patient Compliance in ZETA-1 with Convenient Oral Dosing APX3330 Demonstrated Slowing of Progression of Diabetic
Retinopathy Over 350 Subjects (Healthy, Liver, Cancer, Diabetic) treated with Several Subjects Systemically Dosed ~1 Year and Others at 24-Wks Few Systemic AEs Across All Doses (120mg-720mg) < 5% Mild Skin Rash/Pruritis
(reversible) < 5% Mild Diarrhea No Treatment-Related Organ Toxicity Minimal Ocular Side Effects* APX3330: Well-tolerated Oral Dose up to 600 mg/day | Twice Daily Dosing MOA and Efficacy Signals in DR Favorable Safety
Profile 10 Source: ZETA-1 Clinical Trial *1 subject had vision blur thought to be related by investigator in ZETA-1
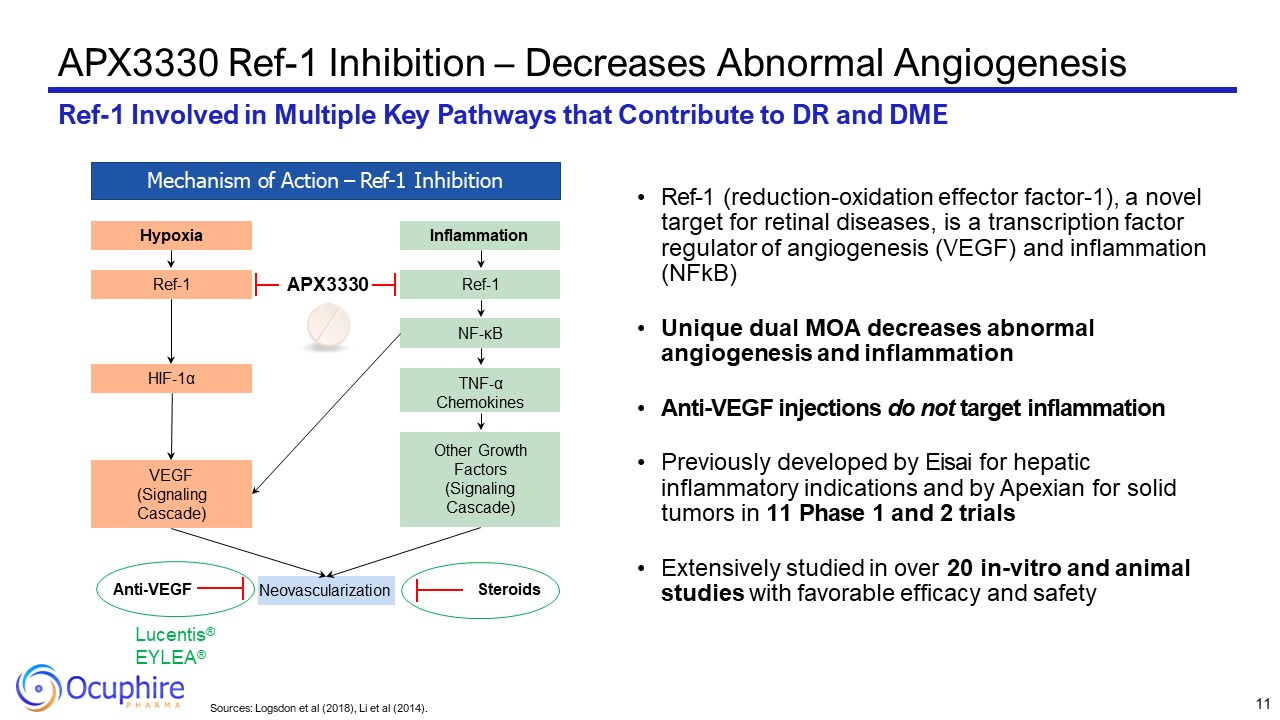
Sources: Logsdon et al (2018), Li et al (2014). APX3330 Ref-1 Inhibition –
Decreases Abnormal Angiogenesis Ref-1 Involved in Multiple Key Pathways that Contribute to DR and DME Mechanism of Action – Ref-1 Inhibition Hypoxia Ref-1 HIF-1α VEGF (Signaling Cascade) Inflammation Ref-1 NF-κB Other Growth
Factors (Signaling Cascade) TNF-α Chemokines Neovascularization Lucentis® EYLEA® Anti-VEGF Steroids APX3330 Ref-1 (reduction-oxidation effector factor-1), a novel target for retinal diseases, is a transcription factor regulator of
angiogenesis (VEGF) and inflammation (NFkB) Unique dual MOA decreases abnormal angiogenesis and inflammation Anti-VEGF injections do not target inflammation Previously developed by Eisai for hepatic inflammatory indications and by Apexian
for solid tumors in 11 Phase 1 and 2 trials Extensively studied in over 20 in-vitro and animal studies with favorable efficacy and safety 11
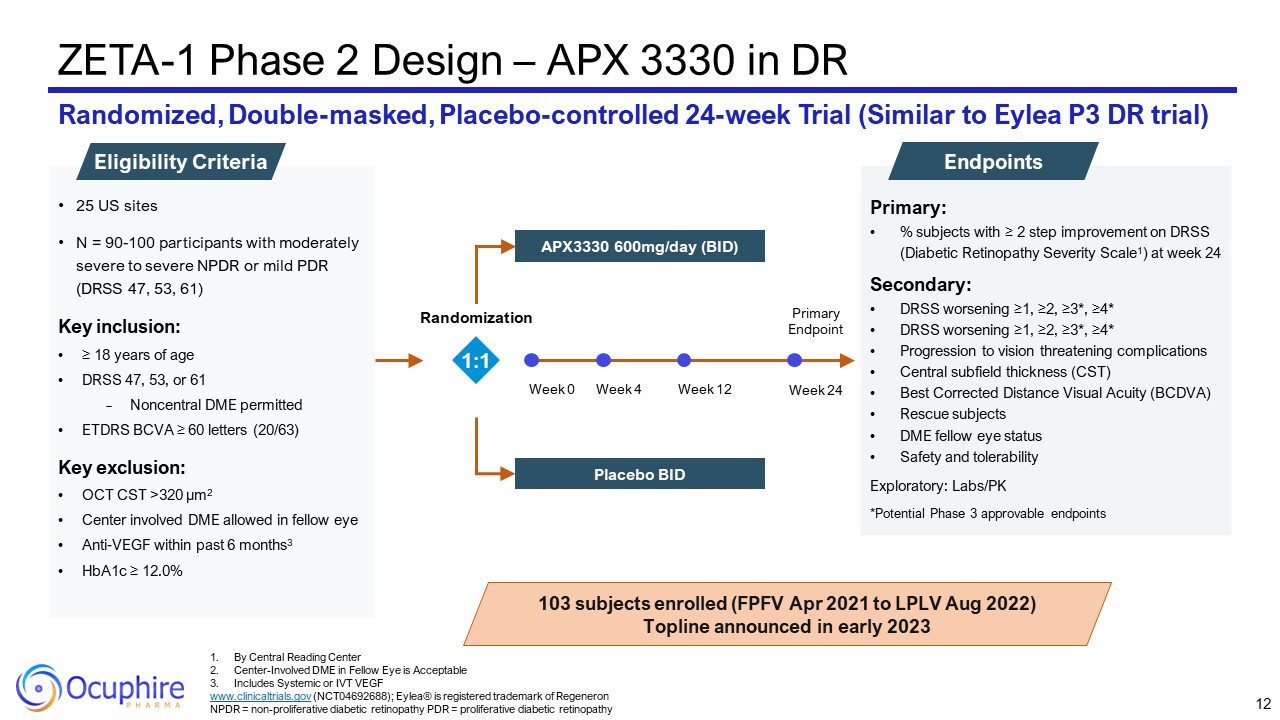
Primary: % subjects with ≥ 2 step improvement on DRSS (Diabetic Retinopathy
Severity Scale1) at week 24 Secondary: DRSS worsening ≥1, ≥2, ≥3*, ≥4* DRSS worsening ≥1, ≥2, ≥3*, ≥4* Progression to vision threatening complications Central subfield thickness (CST) Best Corrected Distance Visual Acuity
(BCDVA) Rescue subjects DME fellow eye status Safety and tolerability Exploratory: Labs/PK *Potential Phase 3 approvable endpoints ZETA-1 Phase 2 Design – APX 3330 in DR Randomized, Double-masked, Placebo-controlled 24-week Trial
(Similar to Eylea P3 DR trial) Endpoints 25 US sites N = 90-100 participants with moderately severe to severe NPDR or mild PDR (DRSS 47, 53, 61) Key inclusion: ≥ 18 years of age DRSS 47, 53, or 61 Noncentral DME permitted ETDRS BCVA ≥
60 letters (20/63) Key exclusion: OCT CST >320 µm2 Center involved DME allowed in fellow eye Anti-VEGF within past 6 months3 HbA1c ≥ 12.0% Eligibility Criteria 103 subjects enrolled (FPFV Apr 2021 to LPLV Aug 2022) Topline
announced in early 2023 1:1 By Central Reading Center Center-Involved DME in Fellow Eye is Acceptable Includes Systemic or IVT VEGF www.clinicaltrials.gov (NCT04692688); Eylea® is registered trademark of Regeneron NPDR =
non-proliferative diabetic retinopathy PDR = proliferative diabetic retinopathy 12 Week 0 Week 12 Week 24 Week 4 Primary Endpoint APX3330 600mg/day (BID) Placebo BID Randomization
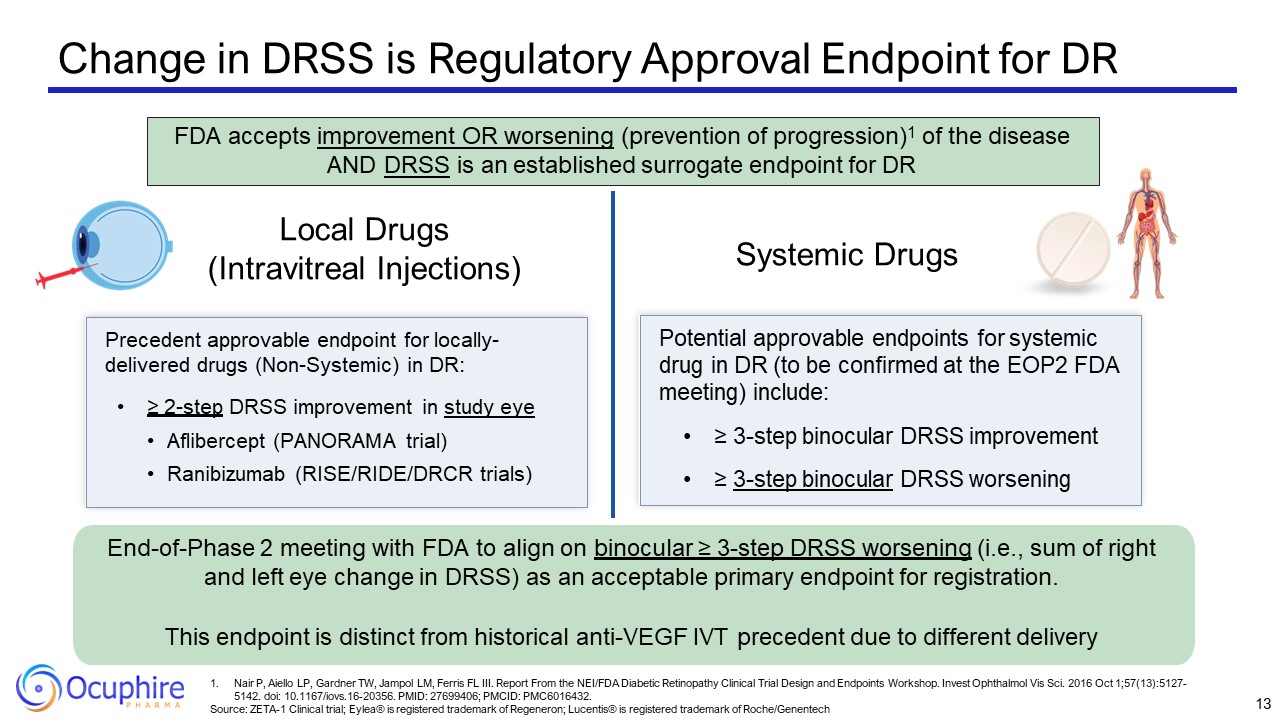
Change in DRSS is Regulatory Approval Endpoint for DR Local Drugs (Intravitreal
Injections) Systemic Drugs Precedent approvable endpoint for locally- delivered drugs (Non-Systemic) in DR: ≥ 2-step DRSS improvement in study eye Aflibercept (PANORAMA trial) Ranibizumab (RISE/RIDE/DRCR trials) Potential approvable
endpoints for systemic drug in DR (to be confirmed at the EOP2 FDA meeting) include: ≥ 3-step binocular DRSS improvement ≥ 3-step binocular DRSS worsening End-of-Phase 2 meeting with FDA to align on binocular ≥ 3-step DRSS worsening (i.e.,
sum of right and left eye change in DRSS) as an acceptable primary endpoint for registration. This endpoint is distinct from historical anti-VEGF IVT precedent due to different delivery FDA accepts improvement OR worsening (prevention of
progression)1 of the disease AND DRSS is an established surrogate endpoint for DR 13 Nair P, Aiello LP, Gardner TW, Jampol LM, Ferris FL III. Report From the NEI/FDA Diabetic Retinopathy Clinical Trial Design and Endpoints Workshop. Invest
Ophthalmol Vis Sci. 2016 Oct 1;57(13):5127-5142. doi: 10.1167/iovs.16-20356. PMID: 27699406; PMCID: PMC6016432. Source: ZETA-1 Clinical trial; Eylea® is registered trademark of Regeneron; Lucentis® is registered trademark of Roche/Genentech
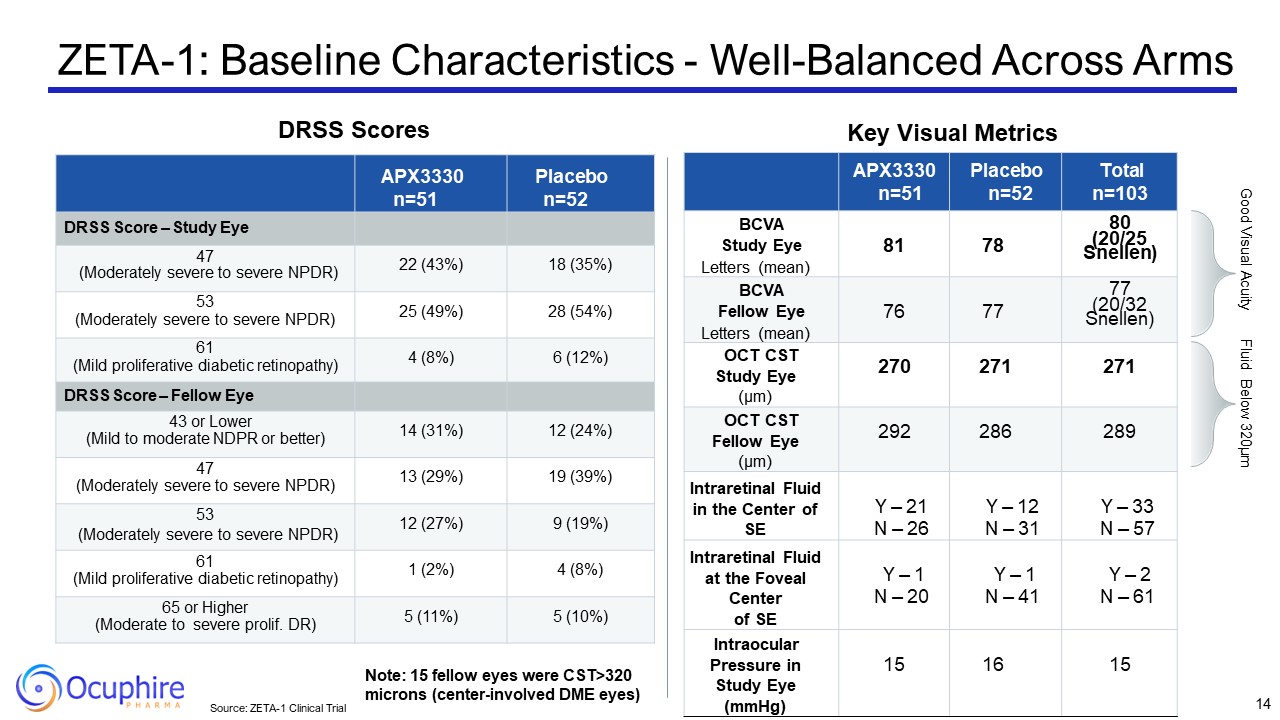
Source: ZETA-1 Clinical Trial Key Visual Metrics APX3330 n=51 Placebo
n=52 Total n=103 BCVA Study Eye Letters (mean) 81 78 80 (20/25 Snellen) BCVA Fellow Eye Letters (mean) 76 77 77 (20/32 Snellen) OCT CST Study Eye (µm) 270 271 271 OCT CST Fellow Eye (µm) 292 286 289 Intraretinal
Fluid in the Center of SE Y – 21 N – 26 Y – 12 N – 31 Y – 33 N – 57 Intraretinal Fluid at the Foveal Center of SE Y – 1 N – 20 Y – 1 N – 41 Y – 2 N – 61 Intraocular Pressure in Study Eye (mmHg) 15 16 15 APX3330 n=51 Placebo
n=52 DRSS Score – Study Eye 47 (Moderately severe to severe NPDR) 22 (43%) 18 (35%) 53 (Moderately severe to severe NPDR) 25 (49%) 28 (54%) 61 (Mild proliferative diabetic retinopathy) 4 (8%) 6 (12%) DRSS Score – Fellow Eye 43 or
Lower (Mild to moderate NDPR or better) 14 (31%) 12 (24%) 47 (Moderately severe to severe NPDR) 13 (29%) 19 (39%) 53 (Moderately severe to severe NPDR) 12 (27%) 9 (19%) 61 (Mild proliferative diabetic retinopathy) 1 (2%) 4
(8%) 65 or Higher (Moderate to severe prolif. DR) 5 (11%) 5 (10%) Good Visual Acuity Fluid Below 320µm Note: 15 fellow eyes were CST>320 microns (center-involved DME eyes) ZETA-1: Baseline Characteristics - Well-Balanced Across
Arms 14 DRSS Scores
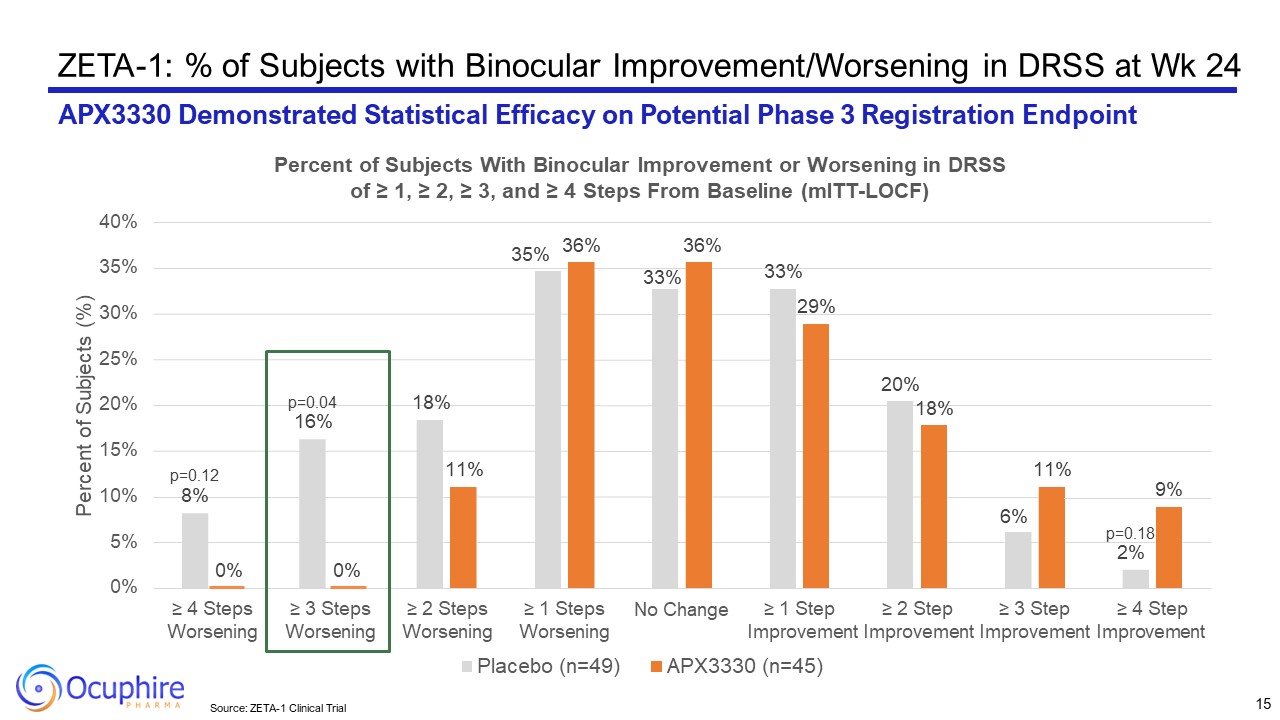
ZETA-1: % of Subjects with Binocular Improvement/Worsening in DRSS at Wk
24 APX3330 Demonstrated Statistical Efficacy on Potential Phase 3 Registration Endpoint p=0.12 8% p=0.04 16% 18% 35% 33% 33% 20% 6% p=0.18 2% 0% 0% 11% 36% 36% 29% 18% 11% 9%
0% 5% 10% 15% 20% 25% 30% 35% 40% ≥ 4 Steps Worsening ≥ 3 Steps Worsening ≥ 2 Steps Worsening ≥ 1 Steps Worsening No Change ≥ 1 Step ≥ 2 Step ≥ 3 Step ≥ 4 Step Improvement Improvement Improvement Improvement Percent of
Subjects (%) Percent of Subjects With Binocular Improvement or Worsening in DRSS of ≥ 1, ≥ 2, ≥ 3, and ≥ 4 Steps From Baseline (mITT-LOCF) Placebo (n=49) APX3330 (n=45) 15 Source: ZETA-1 Clinical Trial
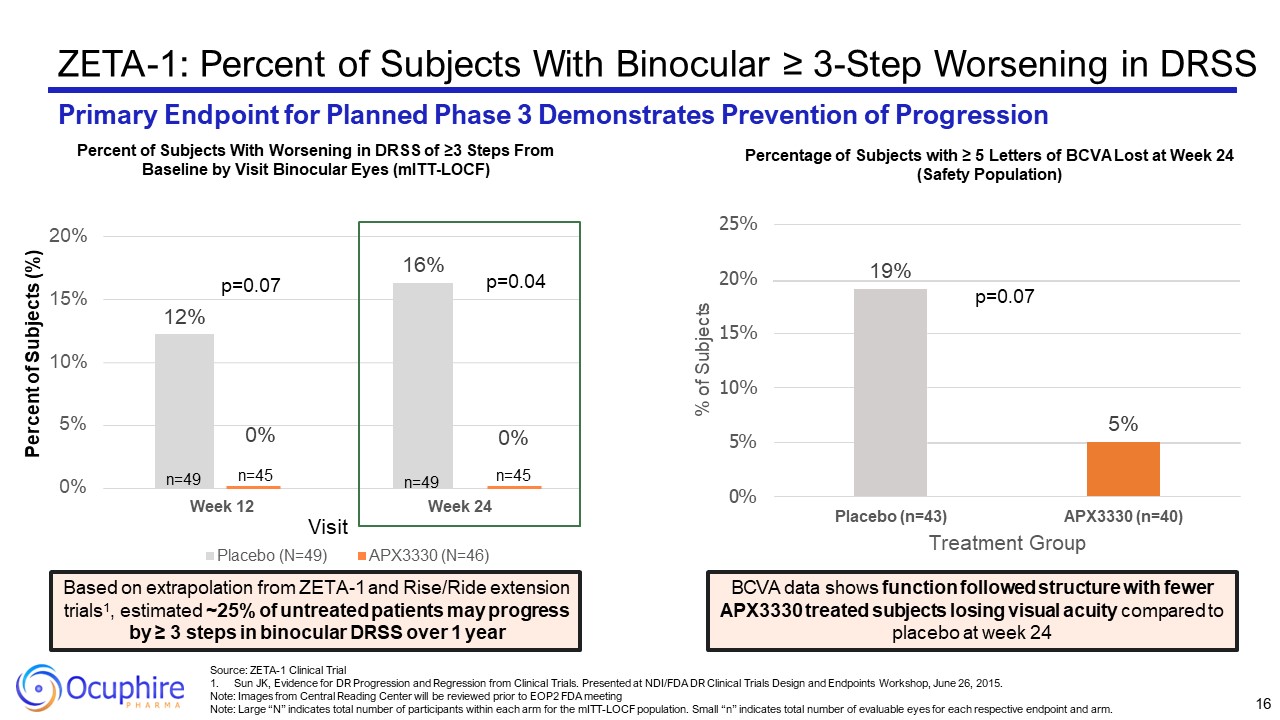
ZETA-1: Percent of Subjects With Binocular ≥ 3-Step Worsening in DRSS Primary
Endpoint for Planned Phase 3 Demonstrates Prevention of Progression 12% 16% 0% 5% 10% 15% 20% Week 12 Week 24 Percent of Subjects (%) Visit Placebo (N=49) APX3330 (N=46) n=49 0% n=45 0% n=45 n=49 p=0.04 p=0.07 Percent of
Subjects With Worsening in DRSS of ≥3 Steps From Baseline by Visit Binocular Eyes (mITT-LOCF) Based on extrapolation from ZETA-1 and Rise/Ride extension trials1, estimated ~25% of untreated patients may progress by ≥ 3 steps in binocular
DRSS over 1 year 5% 19% 0% 5% 10% 15% 20% 25% Placebo (n=43) APX3330 (n=40) Treatment Group % of Subjects p=0.07 BCVA data shows function followed structure with fewer APX3330 treated subjects losing visual acuity compared to
placebo at week 24 Percentage of Subjects with ≥ 5 Letters of BCVA Lost at Week 24 (Safety Population) 16 Source: ZETA-1 Clinical Trial Sun JK, Evidence for DR Progression and Regression from Clinical Trials. Presented at NDI/FDA DR
Clinical Trials Design and Endpoints Workshop, June 26, 2015. Note: Images from Central Reading Center will be reviewed prior to EOP2 FDA meeting Note: Large “N” indicates total number of participants within each arm for the mITT-LOCF
population. Small “n” indicates total number of evaluable eyes for each respective endpoint and arm.
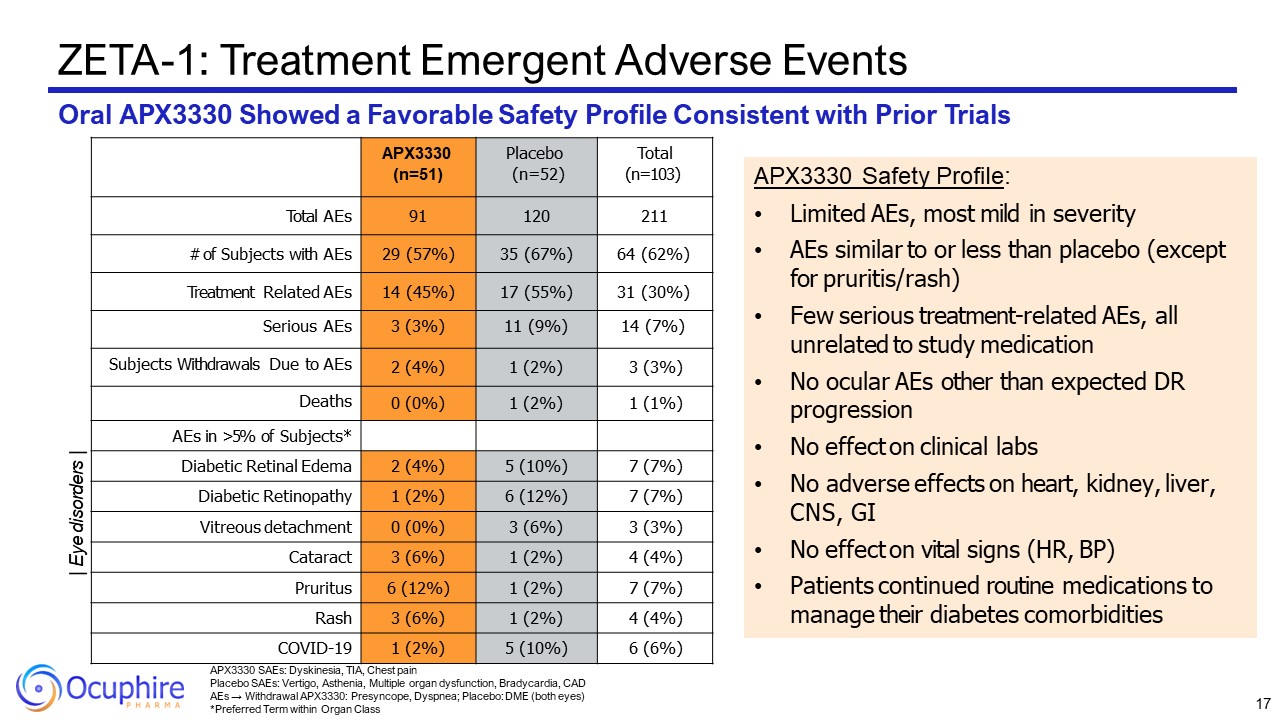
APX3330 SAEs: Dyskinesia, TIA, Chest pain Placebo SAEs: Vertigo, Asthenia,
Multiple organ dysfunction, Bradycardia, CAD AEs → Withdrawal APX3330: Presyncope, Dyspnea; Placebo: DME (both eyes) *Preferred Term within Organ Class ZETA-1: Treatment Emergent Adverse Events Oral APX3330 Showed a Favorable Safety
Profile Consistent with Prior Trials APX3330 (n=51) Placebo (n=52) Total (n=103) Total AEs 91 120 211 # of Subjects with AEs 29 (57%) 35 (67%) 64 (62%) Treatment Related AEs 14 (45%) 17 (55%) 31 (30%) Serious AEs 3 (3%) 11
(9%) 14 (7%) Subjects Withdrawals Due to AEs 2 (4%) 1 (2%) 3 (3%) Deaths 0 (0%) 1 (2%) 1 (1%) AEs in >5% of Subjects* Diabetic Retinal Edema 2 (4%) 5 (10%) 7 (7%) Diabetic Retinopathy 1 (2%) 6 (12%) 7 (7%) Vitreous
detachment 0 (0%) 3 (6%) 3 (3%) Cataract 3 (6%) 1 (2%) 4 (4%) Pruritus 6 (12%) 1 (2%) 7 (7%) Rash 3 (6%) 1 (2%) 4 (4%) COVID-19 1 (2%) 5 (10%) 6 (6%) APX3330 Safety Profile: Limited AEs, most mild in severity AEs similar
to or less than placebo (except for pruritis/rash) Few serious treatment-related AEs, all unrelated to study medication No ocular AEs other than expected DR progression No effect on clinical labs No adverse effects on heart, kidney,
liver, CNS, GI No effect on vital signs (HR, BP) Patients continued routine medications to manage their diabetes comorbidities | Eye disorders | 17
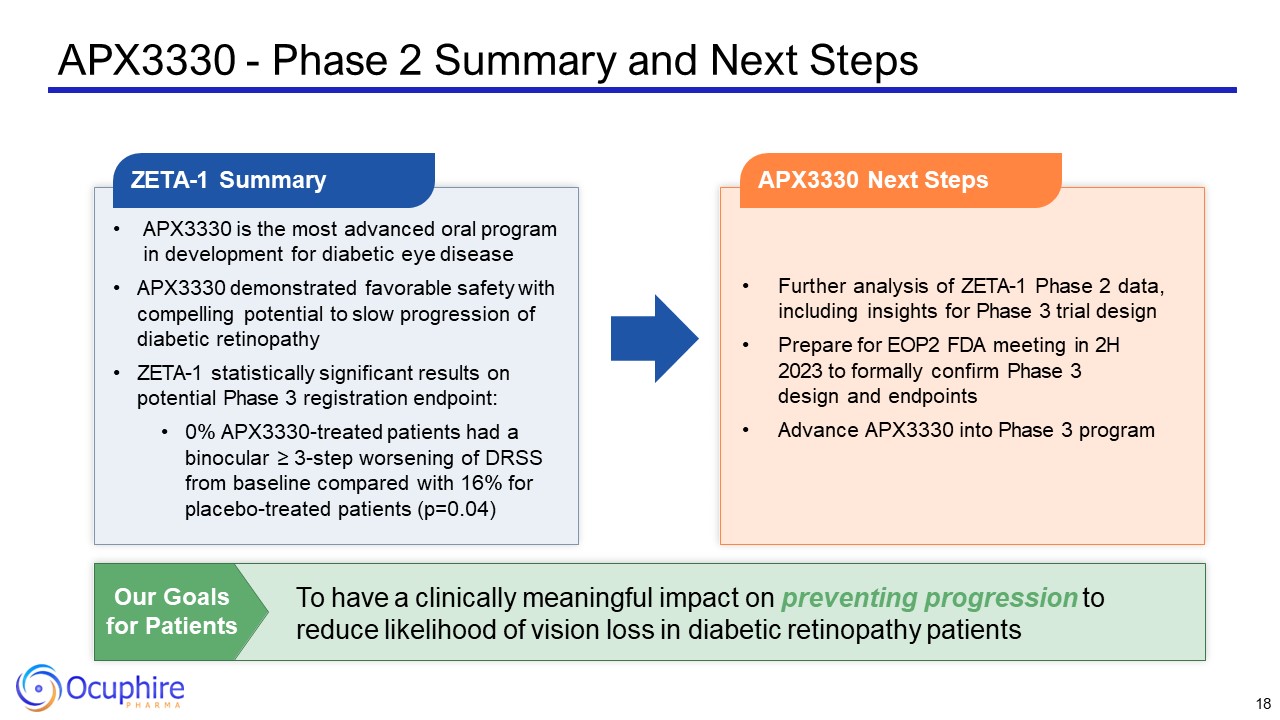
APX3330 - Phase 2 Summary and Next Steps APX3330 is the most advanced oral
program in development for diabetic eye disease APX3330 demonstrated favorable safety with compelling potential to slow progression of diabetic retinopathy ZETA-1 statistically significant results on potential Phase 3 registration
endpoint: 0% APX3330-treated patients had a binocular ≥ 3-step worsening of DRSS from baseline compared with 16% for placebo-treated patients (p=0.04) ZETA-1 Summary Further analysis of ZETA-1 Phase 2 data, including insights for Phase 3
trial design Prepare for EOP2 FDA meeting in 2H 2023 to formally confirm Phase 3 design and endpoints Advance APX3330 into Phase 3 program APX3330 Next Steps To have a clinically meaningful impact on preventing progression to reduce
likelihood of vision loss in diabetic retinopathy patients Our Goals for Patients 18
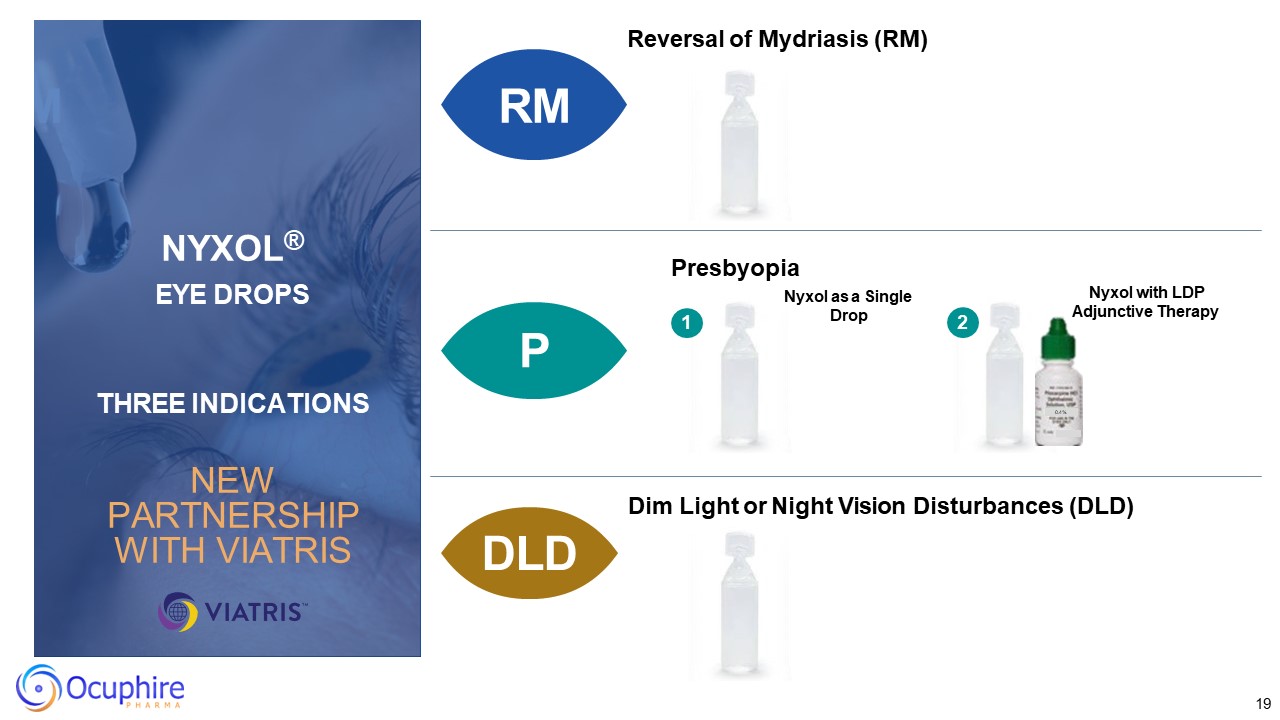
19 Reversal of Mydriasis (RM) P DLD Presbyopia Dim Light or Night Vision
Disturbances (DLD) Nyxol as a Single Drop Nyxol with LDP Adjunctive Therapy 1 0.4% 2 RM RM NYXOL® EYE DROPS THREE INDICATIONS NEW PARTNERSHIP WITH VIATRIS
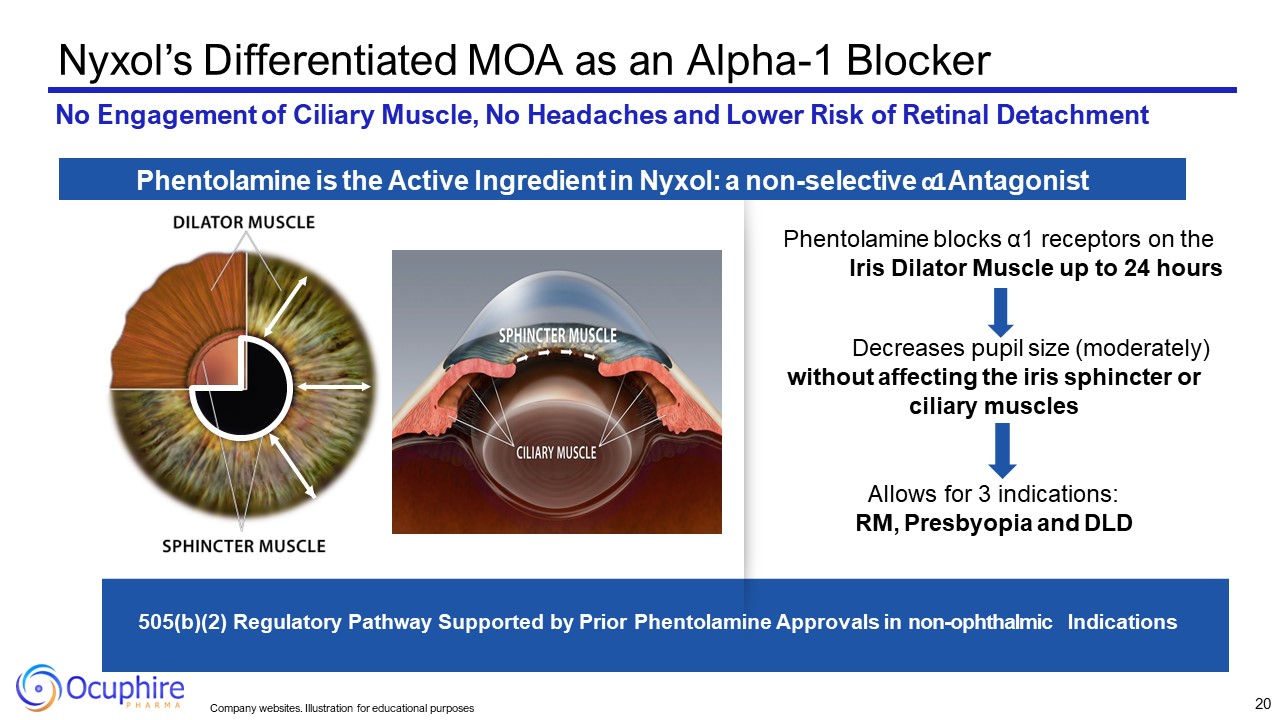
Decreases pupil size (moderately) without affecting the iris sphincter or
ciliary muscles Allows for 3 indications: RM, Presbyopia and DLD 505(b)(2) Regulatory Pathway Supported by Prior Phentolamine Approvals in non-ophthalmic Indications Nyxol’s Differentiated MOA as an Alpha-1 Blocker No Engagement of
Ciliary Muscle, No Headaches and Lower Risk of Retinal Detachment Phentolamine is the Active Ingredient in Nyxol: a non-selective α1 Antagonist Phentolamine blocks α1 receptors on the Iris Dilator Muscle up to 24 hours 20 Company
websites. Illustration for educational purposes
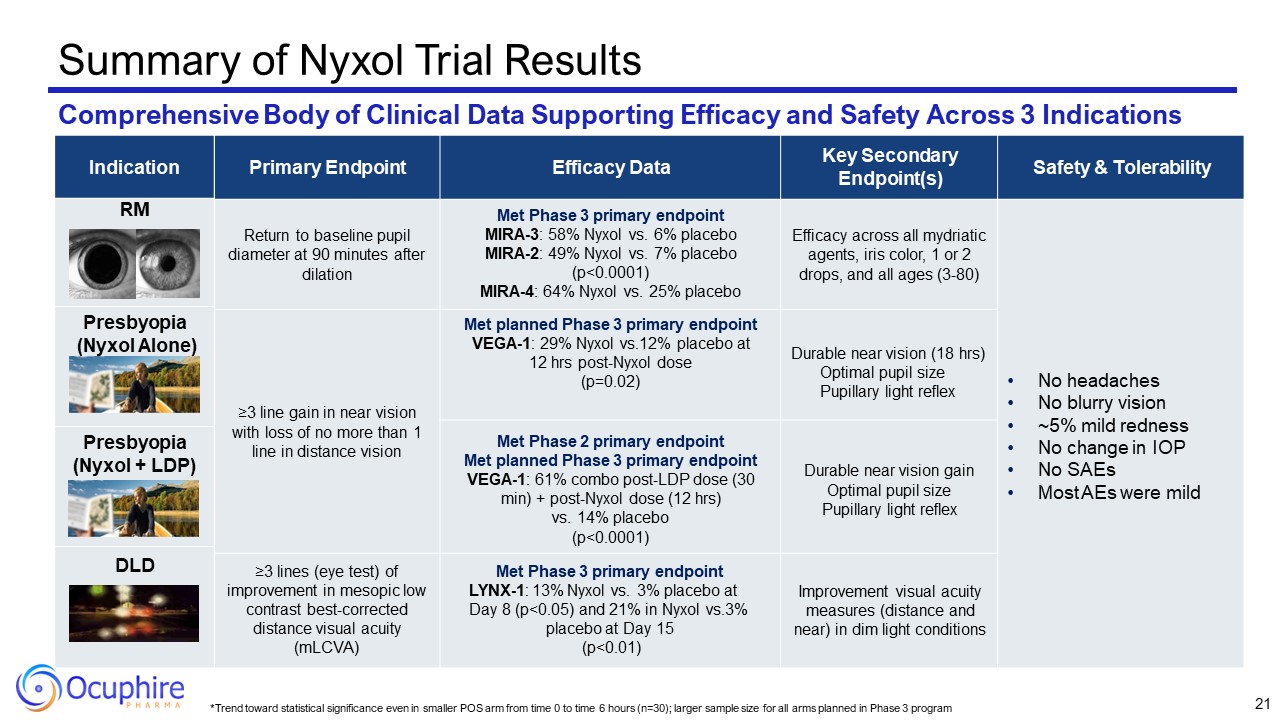
*Trend toward statistical significance even in smaller POS arm from time 0 to
time 6 hours (n=30); larger sample size for all arms planned in Phase 3 program Primary Endpoint Efficacy Data Key Secondary Endpoint(s) Safety & Tolerability Return to baseline pupil diameter at 90 minutes after dilation Met Phase
3 primary endpoint MIRA-3: 58% Nyxol vs. 6% placebo MIRA-2: 49% Nyxol vs. 7% placebo (p<0.0001) MIRA-4: 64% Nyxol vs. 25% placebo Efficacy across all mydriatic agents, iris color, 1 or 2 drops, and all ages (3-80) No headaches No
blurry vision ~5% mild redness No change in IOP No SAEs Most AEs were mild ≥3 line gain in near vision with loss of no more than 1 line in distance vision Met planned Phase 3 primary endpoint VEGA-1: 29% Nyxol vs.12% placebo at 12 hrs
post-Nyxol dose (p=0.02) Durable near vision (18 hrs) Optimal pupil size Pupillary light reflex Met Phase 2 primary endpoint Met planned Phase 3 primary endpoint VEGA-1: 61% combo post-LDP dose (30 min) + post-Nyxol dose (12 hrs) vs. 14%
placebo (p<0.0001) Durable near vision gain Optimal pupil size Pupillary light reflex ≥3 lines (eye test) of improvement in mesopic low contrast best-corrected distance visual acuity (mLCVA) Met Phase 3 primary endpoint LYNX-1: 13%
Nyxol vs. 3% placebo at Day 8 (p<0.05) and 21% in Nyxol vs.3% placebo at Day 15 (p<0.01) Improvement visual acuity measures (distance and near) in dim light conditions Summary of Nyxol Trial Results Comprehensive Body of Clinical
Data Supporting Efficacy and Safety Across 3 Indications RM P: Nyxol DLD 21 Indication RM Presbyopia (Nyxol Alone) Presbyopia (Nyxol + LDP) DLD
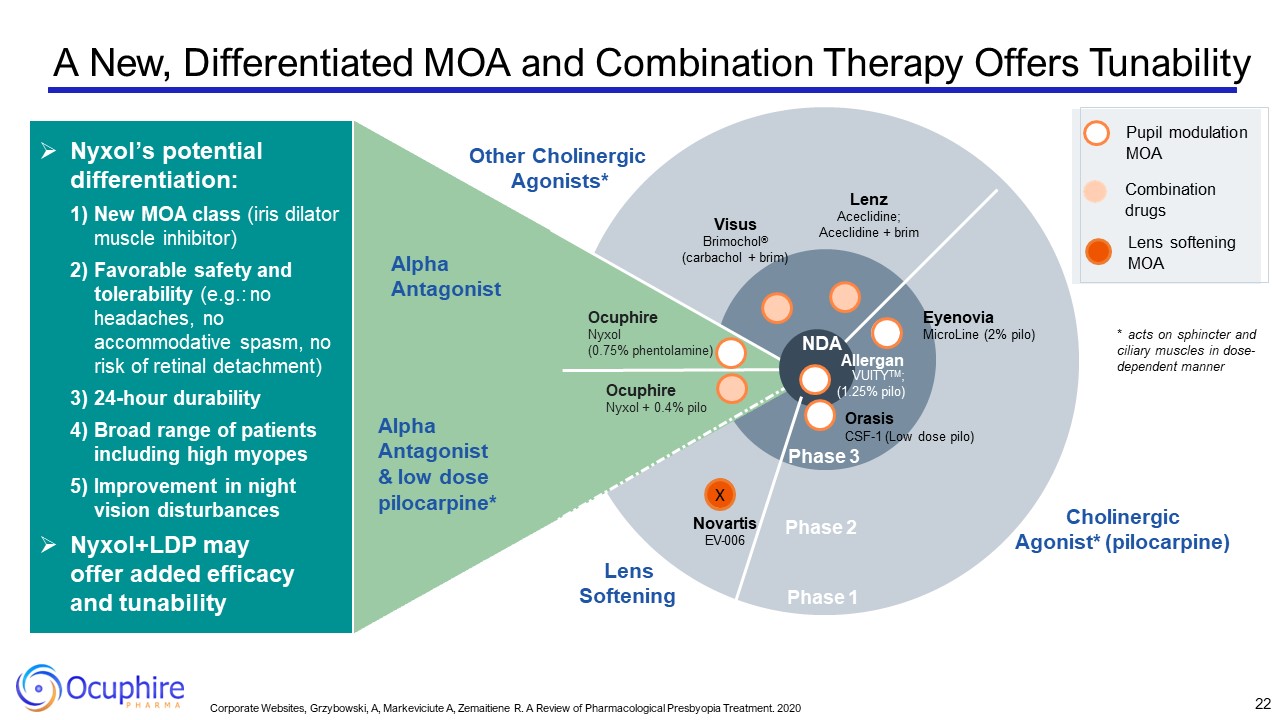
Phase 2 Phase 1 Ocuphire Nyxol + 0.4% pilo Visus Brimochol® (carbachol +
brim) Other Cholinergic Agonists* Cholinergic Agonist* (pilocarpine) Lenz Aceclidine; Aceclidine + brim Eyenovia MicroLine (2% pilo) Alpha Antagonist & low dose pilocarpine* Alpha Antagonist NDA Allergan VUITYTM; (1.25%
pilo) Orasis CSF-1 (Low dose pilo) Phase 3 Nyxol’s potential differentiation: New MOA class (iris dilator muscle inhibitor) Favorable safety and tolerability (e.g.: no headaches, no accommodative spasm, no risk of retinal
detachment) 24-hour durability Broad range of patients including high myopes Improvement in night vision disturbances Nyxol+LDP may offer added efficacy and tunability Ocuphire Nyxol (0.75% phentolamine) Pupil modulation
MOA Combination drugs Lens softening MOA X Novartis EV-006 Lens Softening * acts on sphincter and ciliary muscles in dose- dependent manner 22 Corporate Websites, Grzybowski, A, Markeviciute A, Zemaitiene R. A Review of
Pharmacological Presbyopia Treatment. 2020 A New, Differentiated MOA and Combination Therapy Offers Tunability
Corporate Highlights Two Lead Clinical-Stage Novel Drugs Addressing Multiple
Large Ophthalmology Markets with Limited to No Competition & Extensive Patent Portfolio Global License Agreement with Viatris to Fully Fund the Development and Commercialization of Nyxol for All Indications Strong Financial Position to
Advance APX and Nyxol Clinical Programs into 2025 Nyxol for RM Indication PDUFA Date on September 28, 2023 APX3330 – Paradigm changing oral for 8 million DR patients; Moving into Phase 3 APX3330 oral tablets Diabetic Retinopathy/Diabetic
Macular Edema (DR/DME) Nyxol eyedrops Reversal of Mydriasis (RM) – eye dilation Presbyopia (P) – age-related blurry near vision Dim Light or Night Vision Disturbances (DLD) 23

Restore Vision & Clarity www.ocuphire.com [email protected] Ocuphire
Pharma Appendix: Team and Nyxol Data 24

25 Drey ColemanVP, Clinical Operations Amy Rabourn, CPA SVP, Finance Charlie
Hoffmann, MBA SVP, Corporate Development Mitch Brigell, PhD Head, Clinical Development and Strategy Daniela Oniciu, PhD Global Head, R&D, Chemistry and Product Development Ronil Patel, MS SVP, Operations and BD Chris
Ernst Global Head, QA and Manufacturing Barbara Withers, PhDVP, Clinical and Regulatory Strategy Bindu Manne Head, Market Development and Commercialization Laura Gambino Director, Project Management Richard Rodgers, MBA Interim CEO
Management Team with Decades of Drug Development Experience

Ocuphire's World-Class Medical Advisory Board Chief Medical Advisor, Ocuphire
Refractive Specialist Jay Pepose, MD, PhD UCLA School of Medicine Refractive Specialist Y. Ralph Chu, MD Northwestern University Refractive Specialist Zaina Al-Mohtaseb, MD Baylor College of Medicine Refractive Specialist James Katz, MD
University of Illinois Refractive Specialist Marguerite McDonald, MD Columbia University Refractive Specialist Mitch Jackson, MD University of Chicago Refractive/ Glaucoma Specialist Thomas Samuelson, MD University of
Minnesota Refractive/Glaucoma Specialist Inder Paul Singh, MD The Chicago Medical School Eliot Lazar, MD Georgetown University elCON Medical Retinal Specialist Peter Kaiser, MD Harvard Medical School Retinal Specialist David Lally, MD
Vanderbilt University Retinal Specialist Michael Allingham, MD, PhD University of North Carolina Retinal Specialist David Boyer, MD Chicago Medical School Retinal Specialist David Brown, MD Baylor University Retinal Specialist Jeffrey
Heier, MD Boston University Retinal Specialist Anat Lowenstein, MD, PhD The Hebrew University Retinal Specialist Caroline Baumal, MD University of Toronto Medical School Optometry Douglas Devries, OD University of
Nevada Optometry Paul Karpecki, OD Indiana University Optometry Justin Schweitzer, OD Pacific University College of Optometry Optometry Selina McGee, OD Northeastern State University Optometry Leslie O’Dell, OD Salus
University Co-Founder Apexian/APX3330 Mark Kelley, PhD Indiana University 26

Ocuphire Board of Directors Seasoned Directors with Decades of Drug
Development, M&A/Financings, and Ophthalmology 27 Sean Ainsworth, MBA Lead Independent Director, Board Director Jay Pepose, MD, PhD Board Director Cam Gallagher, MBA Chair, Board Director Mina Sooch, MBA Board Director
Susan Benton, MBA Board Director James Manuso, PhD/MBA Board Director Richard Rodgers, MBA Interim President & CEO Board Director