Exhibit 99.1

ZETA-1 APX3330 Topline Results Investor Webcast January 25, 2023

Disclosures and Forward-Looking Statements This presentation contains
“forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements concerning the success and timing of planned future clinical trials for
APX3330, timing and occurrence of an end of phase 2 meeting with the FDA, the potential of a Phase 3 registration path for APX3330, the success and timing of planned regulatory filings, business strategy, cash runway, scalability, the
potential for APX3330 to be the first line of therapy for DR patients, and the potential market opportunity for the slowing of DR progression. These forward-looking statements are based upon the Company’s current expectations and involve
assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties,
including, without limitation: (i) the success, costs, and timing of regulatory submissions and pre-clinical and clinical trials, including enrollment and data readouts; (ii) regulatory requirements or developments; (iii) changes to clinical
trial designs and regulatory pathways; (iv) changes in capital resource requirements; (v) risks related to the inability of Ocuphire to obtain sufficient additional capital to continue to advance its product candidates and its preclinical
programs; (vi) legislative, regulatory, political and economic developments, (vii) Nyxol partnership may not facilitate the commercialization or market acceptance of Ocuphire’s product candidates; (x) the success and timing of
commercialization of any of Ocuphire’s product candidates, including the scalability of Ocuphire’s product candidates and (xi) the maintenance of Ocuphire’s intellectual property rights. The foregoing review of important factors that could
cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors detailed in documents that have been
and may be filed by the Company from time to time with the SEC. All forward-looking statements contained in this presentation speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to
reflect events that occur or circumstances that exist after the date on which they were made. The Company makes no representation or warranty, express or implied, as to the accuracy or completeness of the information contained in or
incorporated by reference into this presentation. Nothing contained in or incorporated by reference into this presentation is, or shall be relied upon as, a promise or representation by the Company as to the past or future. The Company
assumes no responsibility for the accuracy or completeness of any such information. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market shares and other data about our
industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The trademarks included herein are the property of the owners thereof and are used for reference purposes
only. Such use should not be construed as an endorsement of such products.

Agenda and Speakers Topic ZETA-1 Key Takeaways and APX3330 Oral MOA ZETA-1
Trial Design and Demographics ZETA-1 Efficacy Findings ZETA-1 Safety Findings Overall Summary and Next Steps Q&A Mina Sooch, MBA Founder and Chief Executive Officer Mitch Brigell, PhD Head of Clinical Development and
Strategy Charles Wykoff, MD, PhD Vitreoretinal Specialist Mark Kelley, PhD APX Scientific Founder and Medical Advisor

ZETA-1 Key Takeaways and APX3330 Oral MOA

ZETA-1 Trial: Key Takeaways APX3330 achieved statistical significance on a key
pre-specified secondary endpoint of preventing clinically meaningful progression of diabetic retinopathy (as defined by binocular 3 or more steps worsening on the DRSS1) after 24 weeks of treatment Trend toward more efficacy at 24 weeks vs
12 weeks, suggests that the 52-week Phase 3 trial may generate a larger signal due to an increase in % of placebo subjects who progress Prevention of 3-step worsening (binocular) is a suitable endpoint for an oral, systemically drug
Ocuphire plans to go forward with this potential registration endpoint in Phase 3 following confirmation with the FDA in EOP2 meeting Oral APX3330 demonstrated favorable safety and tolerability Retinal KOLs feedback suggest that slowing of
DR progression with an oral agent would be a useful treatment in patients with background DR and good visual function If approved, APX3330 could be an important new primary preventative therapeutic option that could be used in a large number
of diabetic patients who are earlier in their disease diabetic retinopathy severity scoreSource: ZETA-1 Clinical Trial
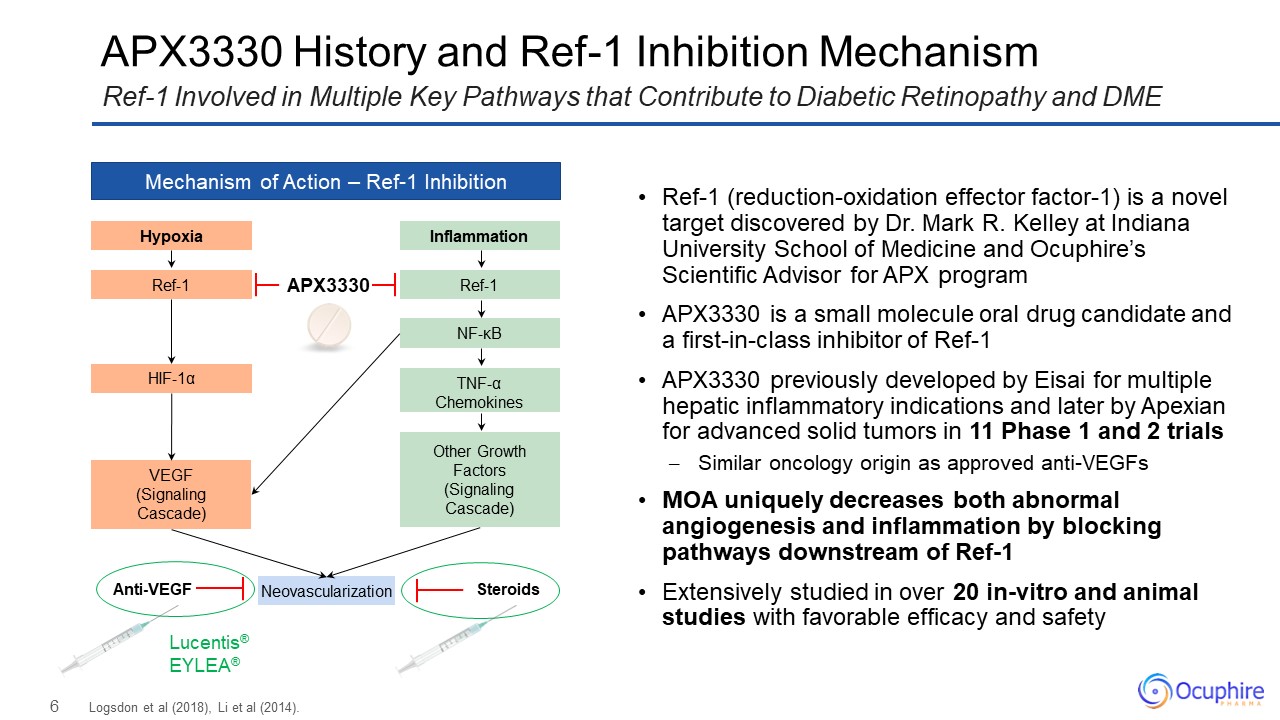
APX3330 History and Ref-1 Inhibition Mechanism Logsdon et al (2018), Li et al
(2014). Ref-1 Involved in Multiple Key Pathways that Contribute to Diabetic Retinopathy and DME Mechanism of Action – Ref-1 Inhibition Hypoxia Ref-1 HIF-1α VEGF (Signaling Cascade) Inflammation Ref-1 NF-κB Other Growth
Factors (Signaling Cascade) TNF-α Chemokines Neovascularization Lucentis® EYLEA® Anti-VEGF Steroids APX3330 Ref-1 (reduction-oxidation effector factor-1) is a novel target discovered by Dr. Mark R. Kelley at Indiana University
School of Medicine and Ocuphire’s Scientific Advisor for APX program APX3330 is a small molecule oral drug candidate and a first-in-class inhibitor of Ref-1 APX3330 previously developed by Eisai for multiple hepatic inflammatory indications
and later by Apexian for advanced solid tumors in 11 Phase 1 and 2 trials Similar oncology origin as approved anti-VEGFs MOA uniquely decreases both abnormal angiogenesis and inflammation by blocking pathways downstream of
Ref-1 Extensively studied in over 20 in-vitro and animal studies with favorable efficacy and safety

ZETA-1 Trial Design and Demographics
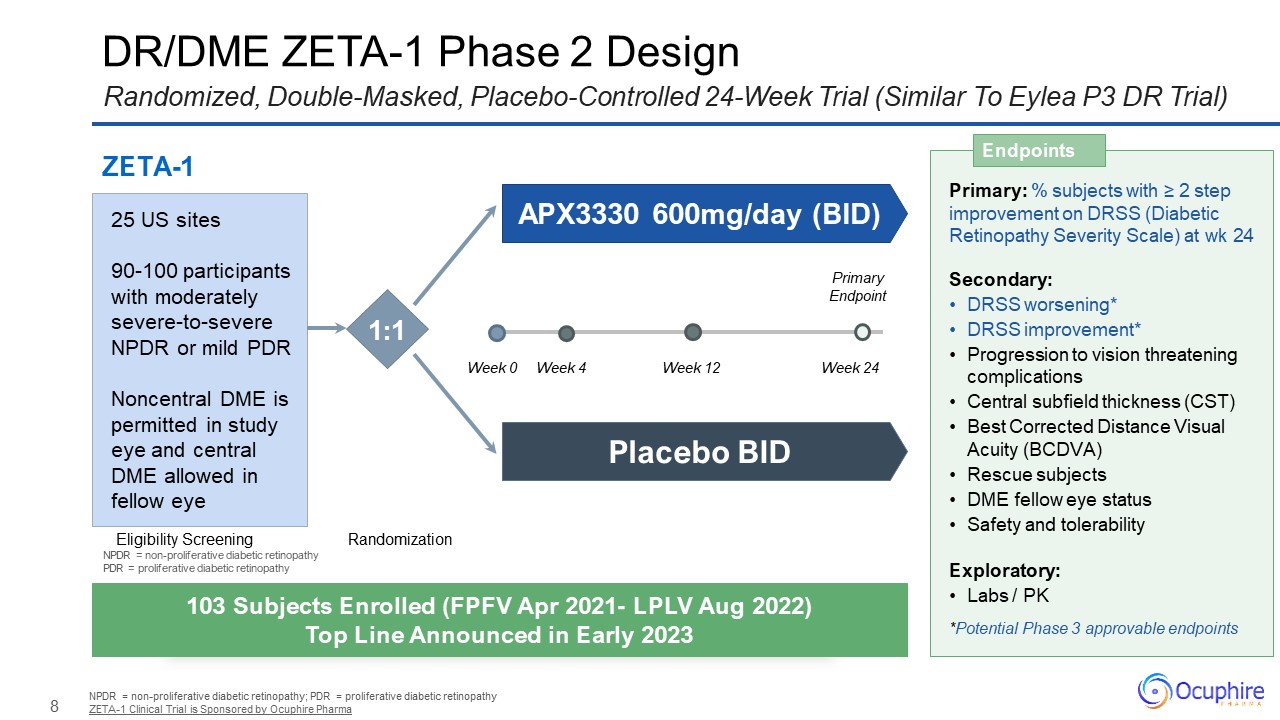
DR/DME ZETA-1 Phase 2 Design NPDR = non-proliferative diabetic retinopathy; PDR
= proliferative diabetic retinopathy ZETA-1 Clinical Trial is Sponsored by Ocuphire Pharma Randomized, Double-Masked, Placebo-Controlled 24-Week Trial (Similar To Eylea P3 DR Trial) Primary: % subjects with ≥ 2 step improvement on DRSS
(Diabetic Retinopathy Severity Scale) at wk 24 Secondary: DRSS worsening* DRSS improvement* Progression to vision threatening complications Central subfield thickness (CST) Best Corrected Distance Visual Acuity (BCDVA) Rescue
subjects DME fellow eye status Safety and tolerability Exploratory: Labs / PK *Potential Phase 3 approvable endpoints Endpoints 103 Subjects Enrolled (FPFV Apr 2021- LPLV Aug 2022) Top Line Announced in Early 2023 ZETA-1 Eligibility
Screening Randomization APX3330 600mg/day (BID) Placebo BID 25 US sites 90-100 participants with moderately severe-to-severe NPDR or mild PDR Noncentral DME is permitted in study eye and central DME allowed in fellow eye 1:1 Week
0 Week 12 Week 24 Week 4 Primary Endpoint NPDR = non-proliferative diabetic retinopathy PDR = proliferative diabetic retinopathy
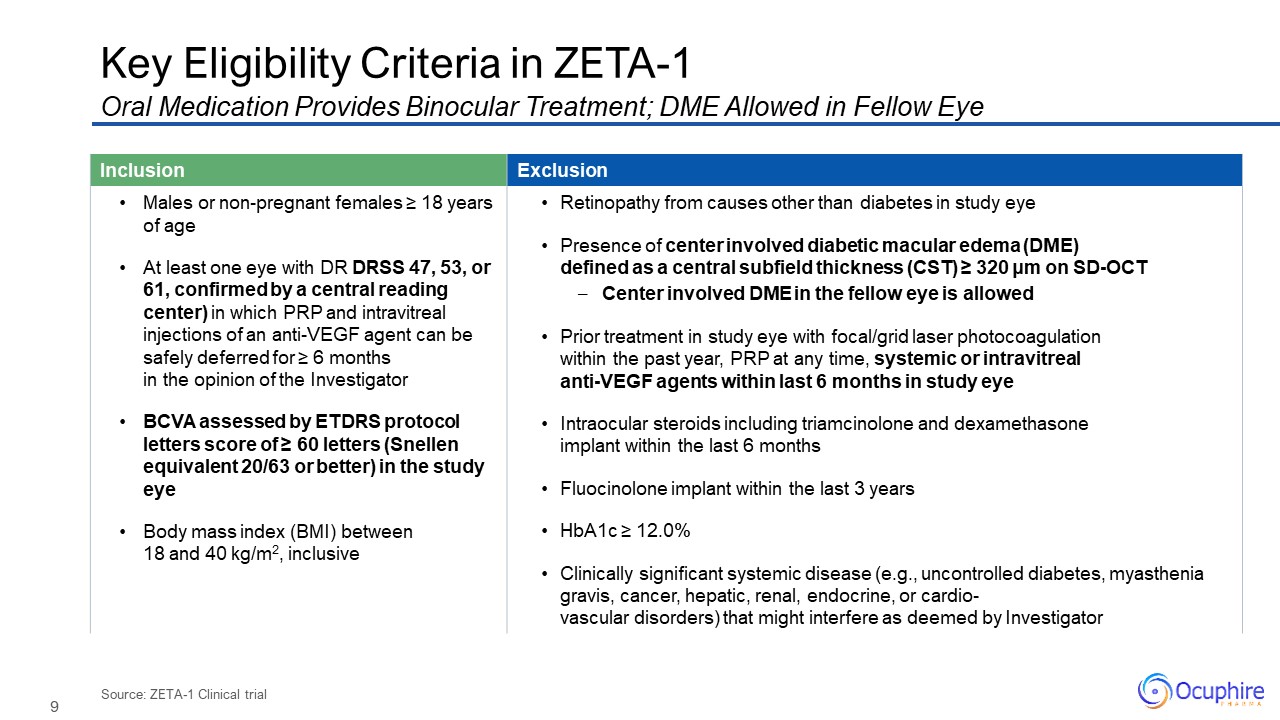
Source: ZETA-1 Clinical trial Key Eligibility Criteria in ZETA-1 Oral
Medication Provides Binocular Treatment; DME Allowed in Fellow Eye Inclusion Exclusion Males or non-pregnant females ≥ 18 years of age At least one eye with DR DRSS 47, 53, or 61, confirmed by a central reading center) in which PRP and
intravitreal injections of an anti-VEGF agent can be safely deferred for ≥ 6 months in the opinion of the Investigator BCVA assessed by ETDRS protocol letters score of ≥ 60 letters (Snellen equivalent 20/63 or better) in the study eye Body
mass index (BMI) between 18 and 40 kg/m2, inclusive Retinopathy from causes other than diabetes in study eye Presence of center involved diabetic macular edema (DME) defined as a central subfield thickness (CST) ≥ 320 μm on SD-OCT Center
involved DME in the fellow eye is allowed Prior treatment in study eye with focal/grid laser photocoagulation within the past year, PRP at any time, systemic or intravitrealanti-VEGF agents within last 6 months in study eye Intraocular
steroids including triamcinolone and dexamethasone implant within the last 6 months Fluocinolone implant within the last 3 years HbA1c ≥ 12.0% Clinically significant systemic disease (e.g., uncontrolled diabetes, myasthenia gravis, cancer,
hepatic, renal, endocrine, or cardio-vascular disorders) that might interfere as deemed by Investigator
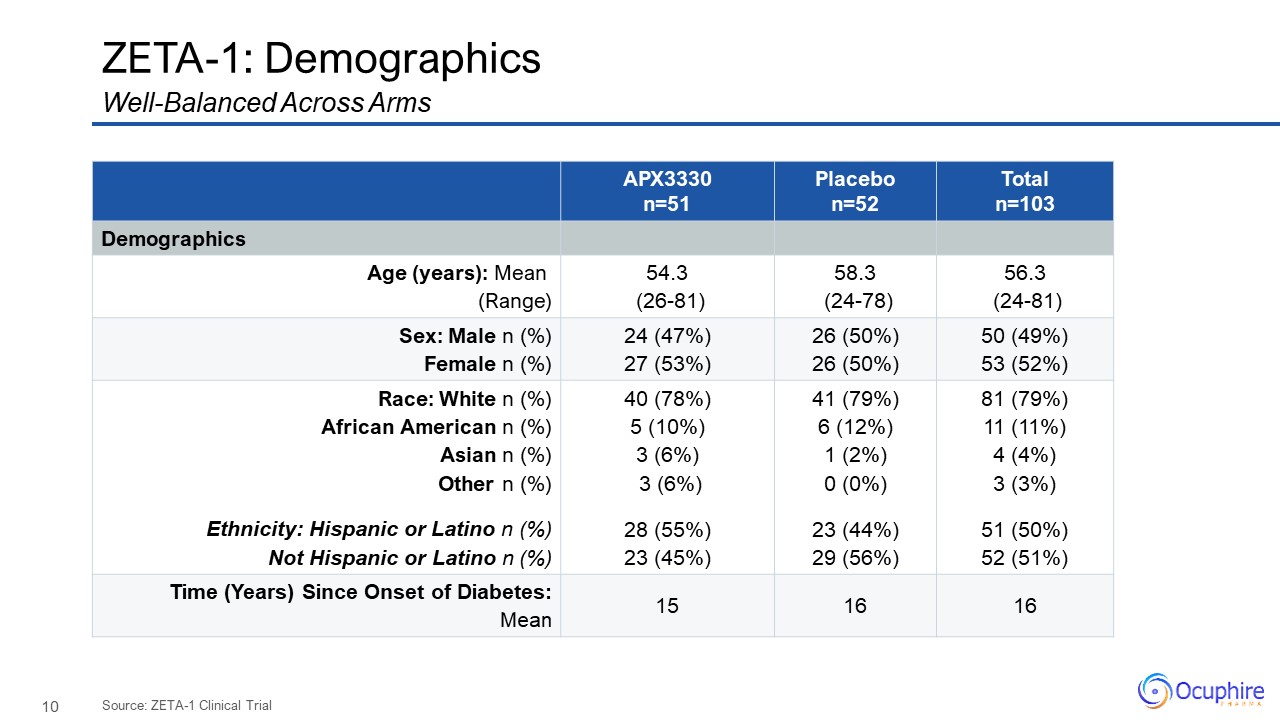
ZETA-1:
Demographics APX3330 n=51 Placebo n=52 Total n=103 Demographics Age (years): Mean (Range) 54.3 (26-81) 58.3 (24-78) 56.3 (24-81) Sex: Male n (%) Female n (%) 24 (47%) 27 (53%) 26 (50%) 26
(50%) 50 (49%) 53 (52%) Race: White n (%) African American n (%) Asian n (%) Other n (%) Ethnicity: Hispanic or Latino n (%) Not Hispanic or Latino n (%) 40 (78%) 5 (10%) 3 (6%) 3 (6%) 28 (55%) 23 (45%) 41
(79%) 6 (12%) 1 (2%) 0 (0%) 23 (44%) 29 (56%) 81 (79%) 11 (11%) 4 (4%) 3 (3%) 51 (50%) 52 (51%) Time (Years) Since Onset of Diabetes:Mean 15 16 16 Well-Balanced Across Arms Source: ZETA-1 Clinical Trial
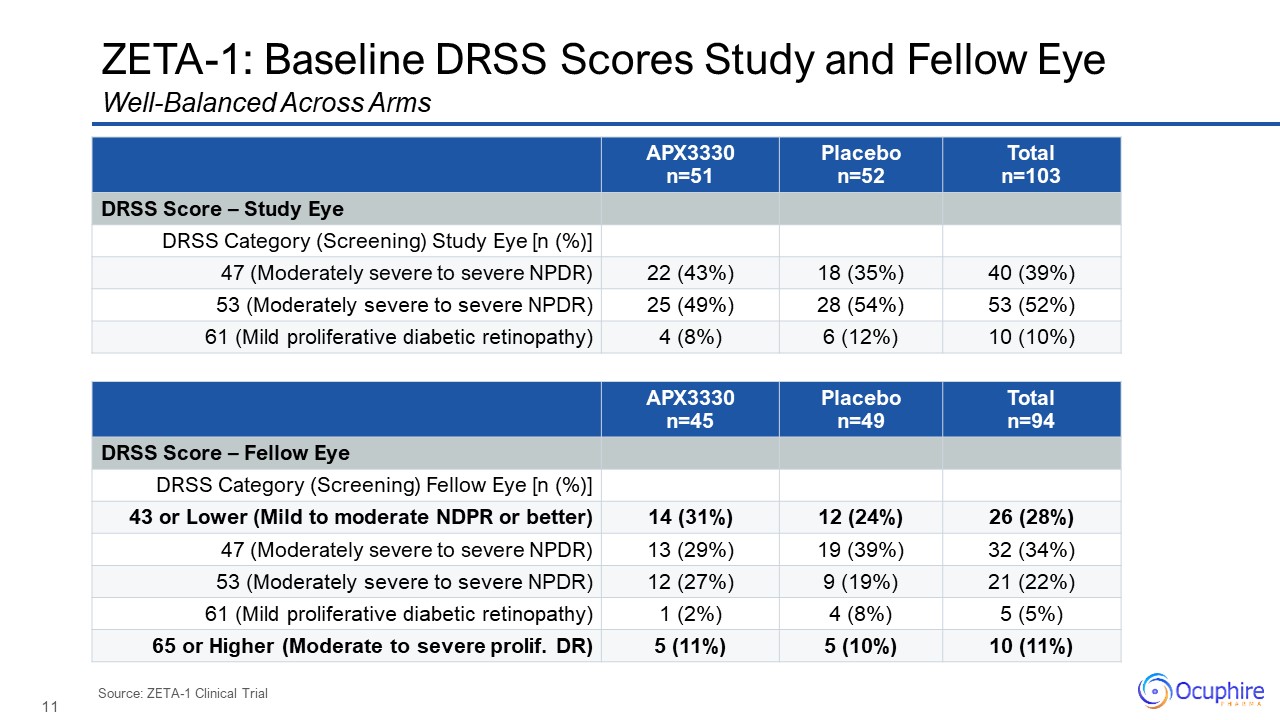
ZETA-1: Baseline DRSS Scores Study and Fellow Eye
APX3330 n=51 Placebo n=52 Total n=103 DRSS Score – Study Eye DRSS Category (Screening) Study Eye [n (%)] 47 (Moderately severe to severe NPDR) 22 (43%) 18 (35%) 40 (39%) 53 (Moderately severe to severe
NPDR) 25 (49%) 28 (54%) 53 (52%) 61 (Mild proliferative diabetic retinopathy) 4 (8%) 6 (12%) 10 (10%) Well-Balanced Across Arms Source: ZETA-1 Clinical Trial APX3330 n=45 Placebo n=49 Total n=94 DRSS Score – Fellow
Eye DRSS Category (Screening) Fellow Eye [n (%)] 43 or Lower (Mild to moderate NDPR or better) 14 (31%) 12 (24%) 26 (28%) 47 (Moderately severe to severe NPDR) 13 (29%) 19 (39%) 32 (34%) 53 (Moderately severe to severe
NPDR) 12 (27%) 9 (19%) 21 (22%) 61 (Mild proliferative diabetic retinopathy) 1 (2%) 4 (8%) 5 (5%) 65 or Higher (Moderate to severe prolif. DR) 5 (11%) 5 (10%) 10 (11%)
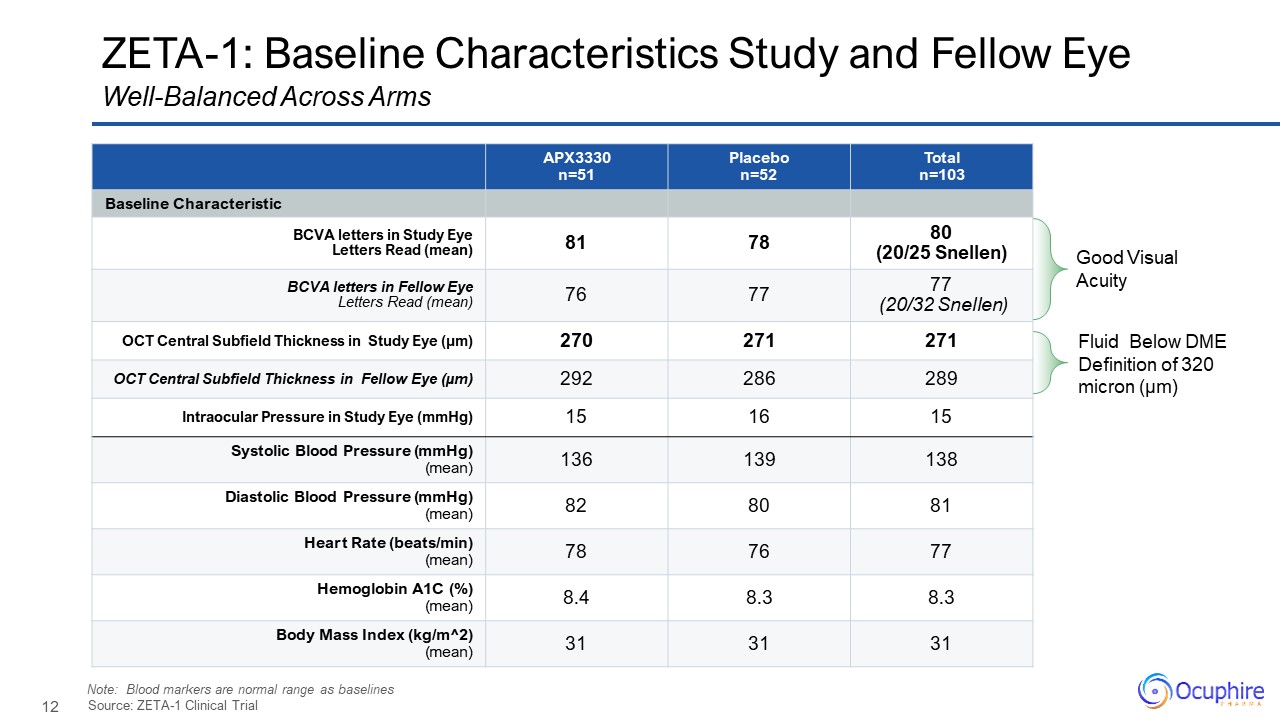
ZETA-1: Baseline Characteristics Study and Fellow
Eye APX3330 n=51 Placebo n=52 Total n=103 Baseline Characteristic BCVA letters in Study Eye Letters Read (mean) 81 78 80 (20/25 Snellen) BCVA letters in Fellow Eye Letters Read (mean) 76 77 77 (20/32 Snellen) OCT Central
Subfield Thickness in Study Eye (µm) 270 271 271 OCT Central Subfield Thickness in Fellow Eye (µm) 292 286 289 Intraocular Pressure in Study Eye (mmHg) 15 16 15 Systolic Blood Pressure (mmHg) (mean) 136 139 138 Diastolic
Blood Pressure (mmHg) (mean) 82 80 81 Heart Rate (beats/min) (mean) 78 76 77 Hemoglobin A1C (%)(mean) 8.4 8.3 8.3 Body Mass Index (kg/m^2)(mean) 31 31 31 Source: ZETA-1 Clinical Trial Well-Balanced Across Arms Good Visual
Acuity Fluid Below DME Definition of 320 micron (µm) Note: Blood markers are normal range as baselines

ZETA-1 Efficacy Findings
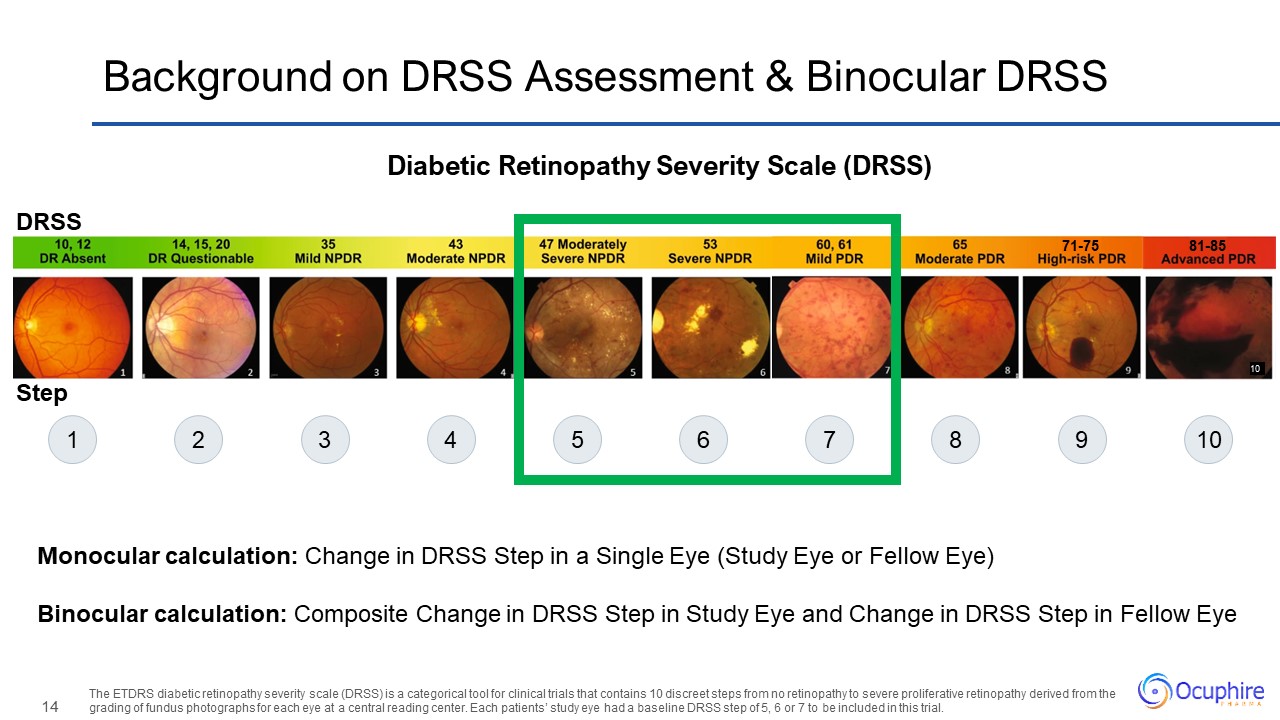
10 81-85 71-75 Background on DRSS Assessment & Binocular DRSS Diabetic
Retinopathy Severity Scale (DRSS) The ETDRS diabetic retinopathy severity scale (DRSS) is a categorical tool for clinical trials that contains 10 discreet steps from no retinopathy to severe proliferative retinopathy derived from the grading
of fundus photographs for each eye at a central reading center. Each patients’ study eye had a baseline DRSS step of 5, 6 or 7 to be included in this trial. Monocular calculation: Change in DRSS Step in a Single Eye (Study Eye or Fellow
Eye) Binocular calculation: Composite Change in DRSS Step in Study Eye and Change in DRSS Step in Fellow Eye 1 2 3 4 5 6 7 8 9 10 Step DRSS
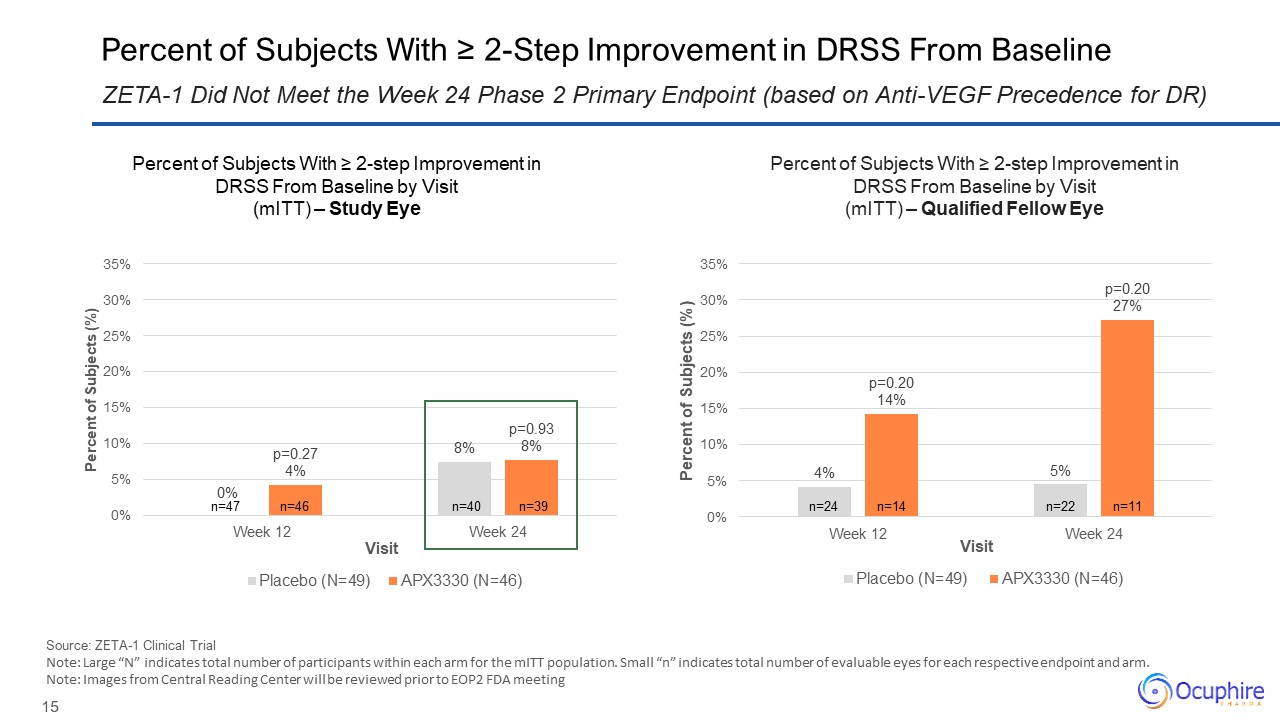
Percent of Subjects With ≥ 2-Step Improvement in DRSS From Baseline Source:
ZETA-1 Clinical Trial Note: Large “N” indicates total number of participants within each arm for the mITT population. Small “n” indicates total number of evaluable eyes for each respective endpoint and arm. Note: Images from Central
Reading Center will be reviewed prior to EOP2 FDA meeting ZETA-1 Did Not Meet the Week 24 Phase 2 Primary Endpoint (based on Anti-VEGF Precedence for DR) n=46 n=47 n=39 n=40 n=14 n=24 n=11 n=22 Percent of Subjects With ≥ 2-step
Improvement in DRSS From Baseline by Visit (mITT) – Study Eye Percent of Subjects With ≥ 2-step Improvement in DRSS From Baseline by Visit (mITT) – Qualified Fellow Eye
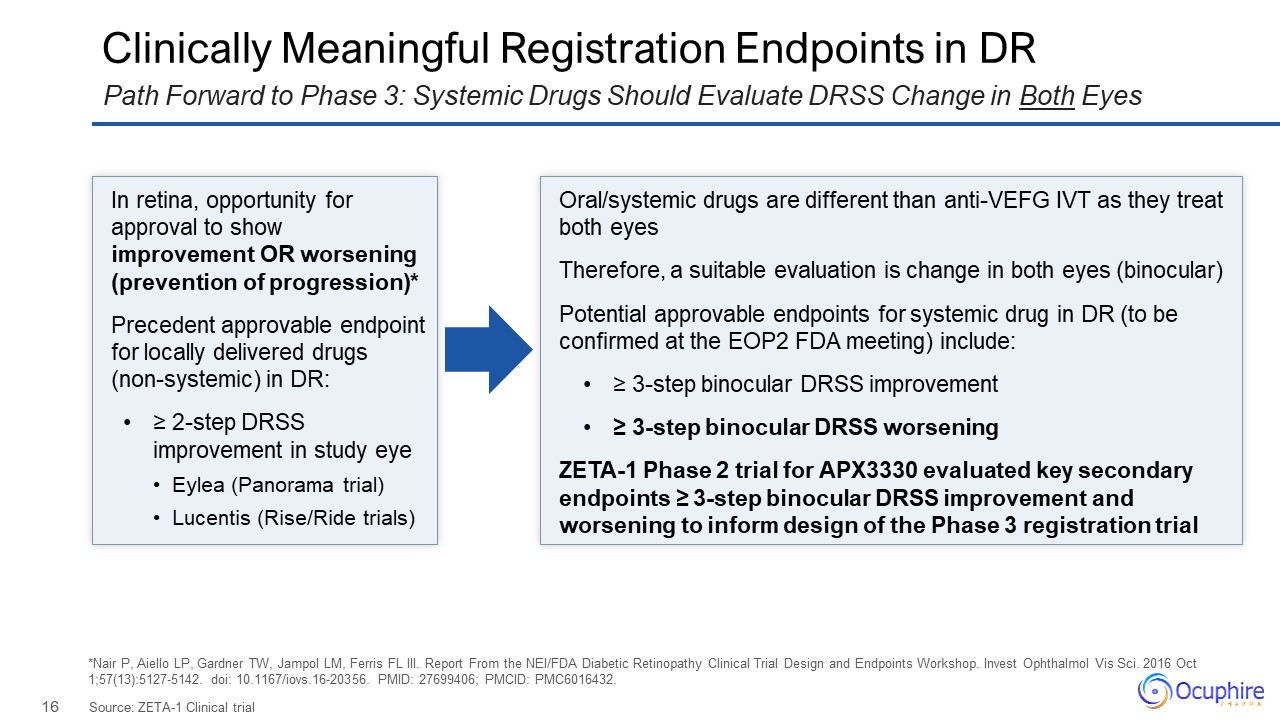
In retina, opportunity for approval to show improvement OR worsening (prevention
of progression)* Precedent approvable endpoint for locally delivered drugs (non-systemic) in DR: ≥ 2-step DRSS improvement in study eye Eylea (Panorama trial) Lucentis (Rise/Ride trials) Oral/systemic drugs are different than anti-VEFG
IVT as they treat both eyes Therefore, a suitable evaluation is change in both eyes (binocular) Potential approvable endpoints for systemic drug in DR (to be confirmed at the EOP2 FDA meeting) include: ≥ 3-step binocular DRSS
improvement ≥ 3-step binocular DRSS worsening ZETA-1 Phase 2 trial for APX3330 evaluated key secondary endpoints ≥ 3-step binocular DRSS improvement and worsening to inform design of the Phase 3 registration trial Clinically Meaningful
Registration Endpoints in DR Source: ZETA-1 Clinical trial Path Forward to Phase 3: Systemic Drugs Should Evaluate DRSS Change in Both Eyes *Nair P, Aiello LP, Gardner TW, Jampol LM, Ferris FL III. Report From the NEI/FDA Diabetic
Retinopathy Clinical Trial Design and Endpoints Workshop. Invest Ophthalmol Vis Sci. 2016 Oct 1;57(13):5127-5142. doi: 10.1167/iovs.16-20356. PMID: 27699406; PMCID: PMC6016432.
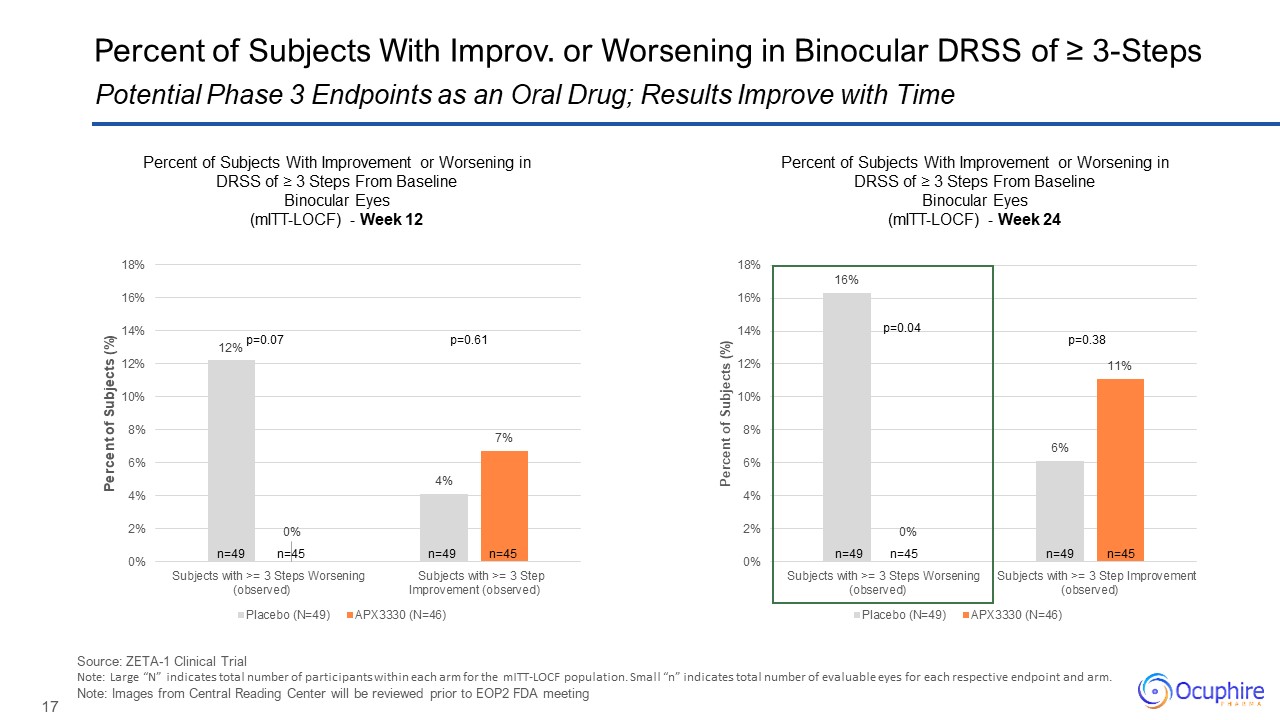
Source: ZETA-1 Clinical Trial Note: Large “N” indicates total number of
participants within each arm for the mITT-LOCF population. Small “n” indicates total number of evaluable eyes for each respective endpoint and arm. Note: Images from Central Reading Center will be reviewed prior to EOP2 FDA meeting
Potential Phase 3 Endpoints as an Oral Drug; Results Improve with Time Percent of Subjects With Improv. or Worsening in Binocular DRSS of ≥ 3-Steps n=49 n=49 n=45 n=45 p=0.04 p=0.38 n=49 n=49 n=45 n=45 p=0.07 p=0.61 Percent
of Subjects With Improvement or Worsening in DRSS of ≥ 3 Steps From Baseline Binocular Eyes (mITT-LOCF) - Week 12 Percent of Subjects With Improvement or Worsening in DRSS of ≥ 3 Steps From Baseline Binocular Eyes (mITT-LOCF) - Week
24
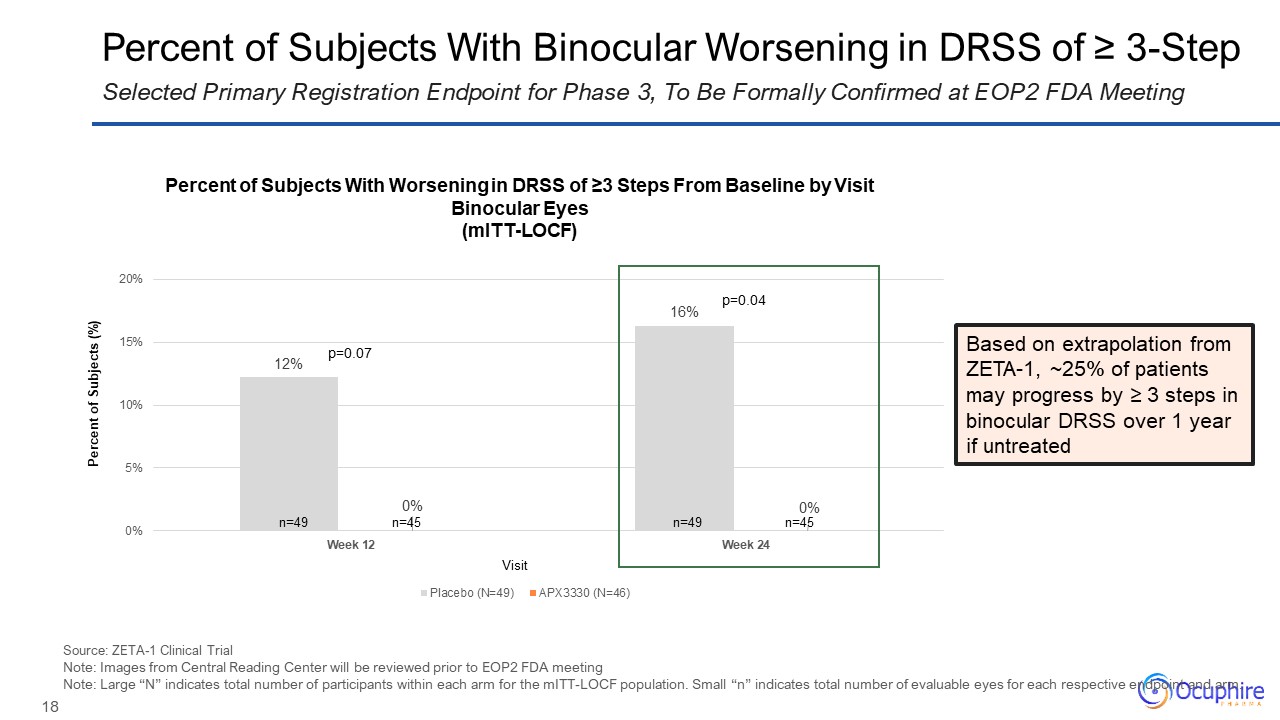
Percent of Subjects With Binocular Worsening in DRSS of ≥ 3-Step Selected
Primary Registration Endpoint for Phase 3, To Be Formally Confirmed at EOP2 FDA Meeting p=0.04 p=0.07 Percent of Subjects With Worsening in DRSS of ≥3 Steps From Baseline by Visit Binocular Eyes (mITT-LOCF) n=49 n=45 n=49 n=45
Source: ZETA-1 Clinical Trial Note: Images from Central Reading Center will be reviewed prior to EOP2 FDA meeting Note: Large “N” indicates total number of participants within each arm for the mITT-LOCF population. Small “n” indicates
total number of evaluable eyes for each respective endpoint and arm. Based on extrapolation from ZETA-1, ~25% of patients may progress by ≥ 3 steps in binocular DRSS over 1 year if untreated
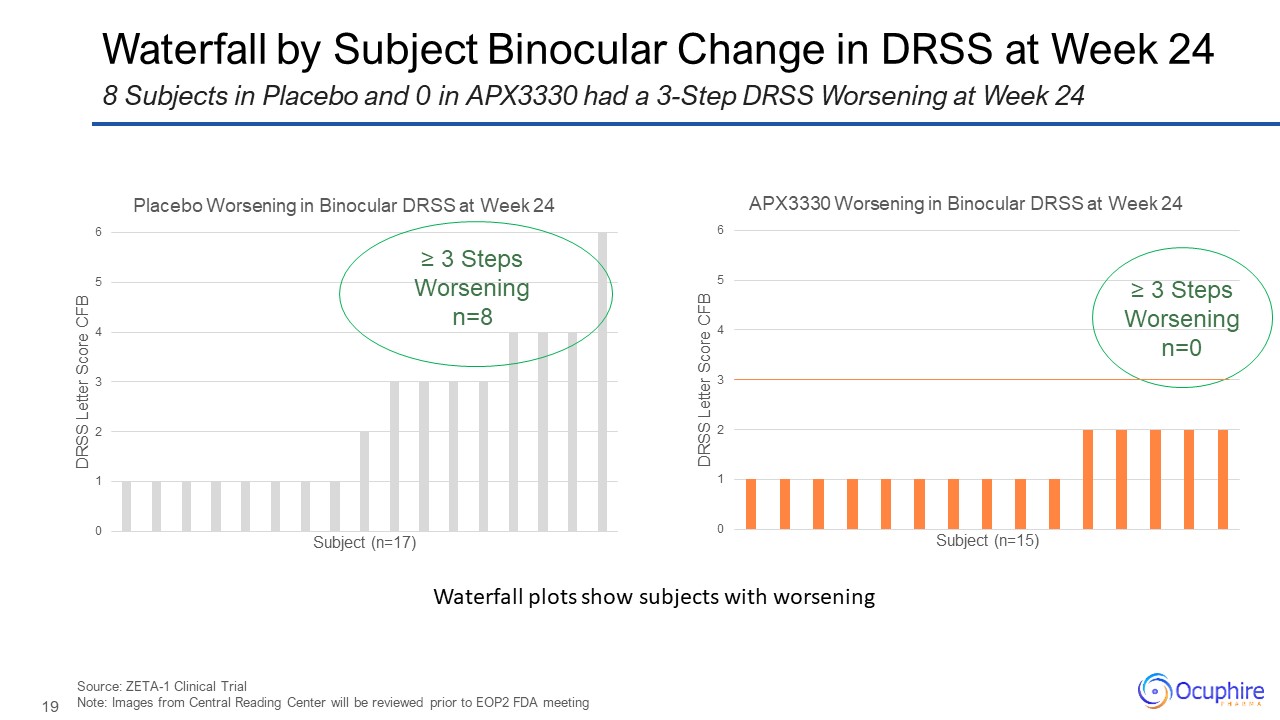
Waterfall by Subject Binocular Change in DRSS at Week 24 Source: ZETA-1
Clinical Trial Note: Images from Central Reading Center will be reviewed prior to EOP2 FDA meeting 8 Subjects in Placebo and 0 in APX3330 had a 3-Step DRSS Worsening at Week 24 ≥ 3 Steps Worsening n=8 ≥ 3 Steps Worsening n=0
Waterfall plots show subjects with worsening
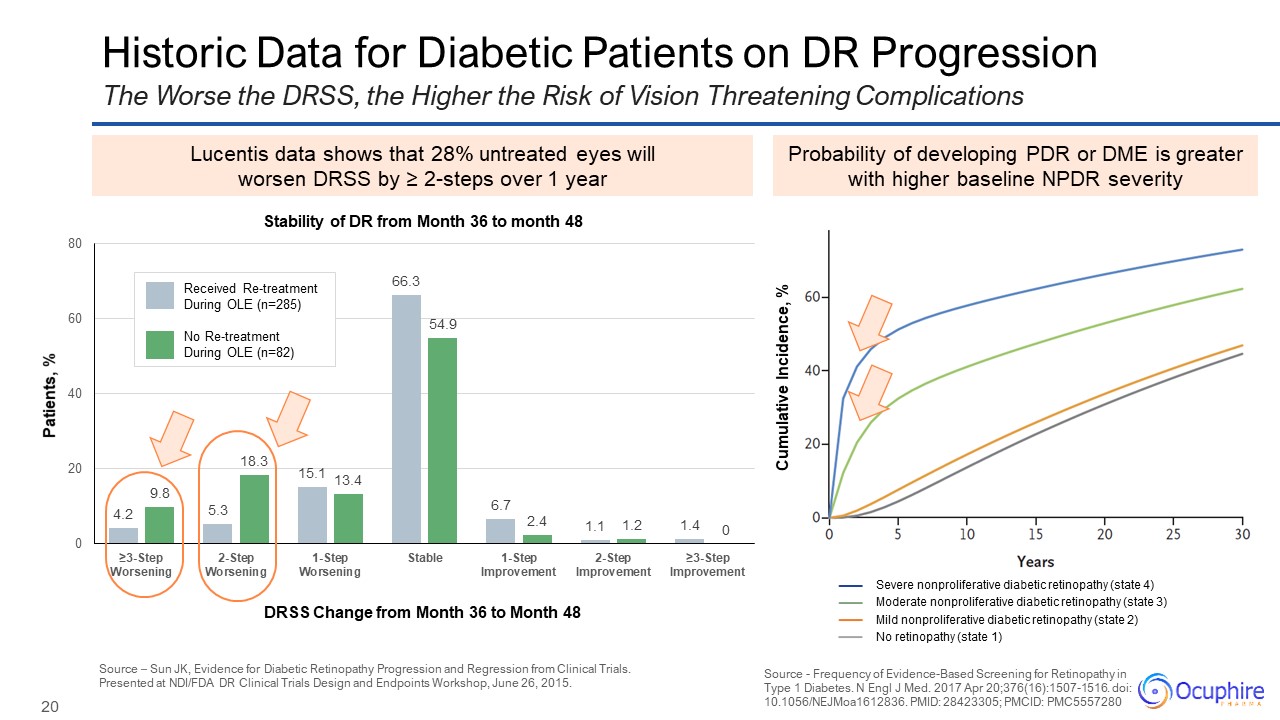
Historic Data for Diabetic Patients on DR Progression Source - Frequency of
Evidence-Based Screening for Retinopathy in Type 1 Diabetes. N Engl J Med. 2017 Apr 20;376(16):1507-1516. doi: 10.1056/NEJMoa1612836. PMID: 28423305; PMCID: PMC5557280 The Worse the DRSS, the Higher the Risk of Vision Threatening
Complications Probability of developing PDR or DME is greater with higher baseline NPDR severity Lucentis data shows that 28% untreated eyes will worsen DRSS by ≥ 2-steps over 1 year Source – Sun JK, Evidence for Diabetic Retinopathy
Progression and Regression from Clinical Trials. Presented at NDI/FDA DR Clinical Trials Design and Endpoints Workshop, June 26, 2015. DRSS Change from Month 36 to Month 48 Patients, % Received Re-treatment During OLE (n=285) No
Re-treatment During OLE (n=82) Stability of DR from Month 36 to month 48 Severe nonproliferative diabetic retinopathy (state 4) Moderate nonproliferative diabetic retinopathy (state 3) Mild nonproliferative diabetic retinopathy (state
2) No retinopathy (state 1) Cumulative Incidence, %

ZETA-1 Safety Findings
PK/Safety Data Findings APX3330 PK/serum levels as predicted at 600
mg/day Serum levels of APX3330 are consistent with previous findings in hepatitis and oncology trials Fewer subjects lost 5 or more letters at week 24 with APX3330 compared to placebo Limited treatment related AEs (mostly mild and
transient) Only rash (6% APX3330 vs 2% placebo) and pruritus (12% APX3330 vs 2% placebo) were seen more frequently in APX3330 than placebo No treatment related serious TEAES No effect on vital signs (BP, HR) No effect on physical
exam No change in liver, kidney, or heart functions No effect on IOP No effect on clinical labs ZETA-1 Clinical Trial (Safety Population) Favorable Safety Data for Oral APX3330
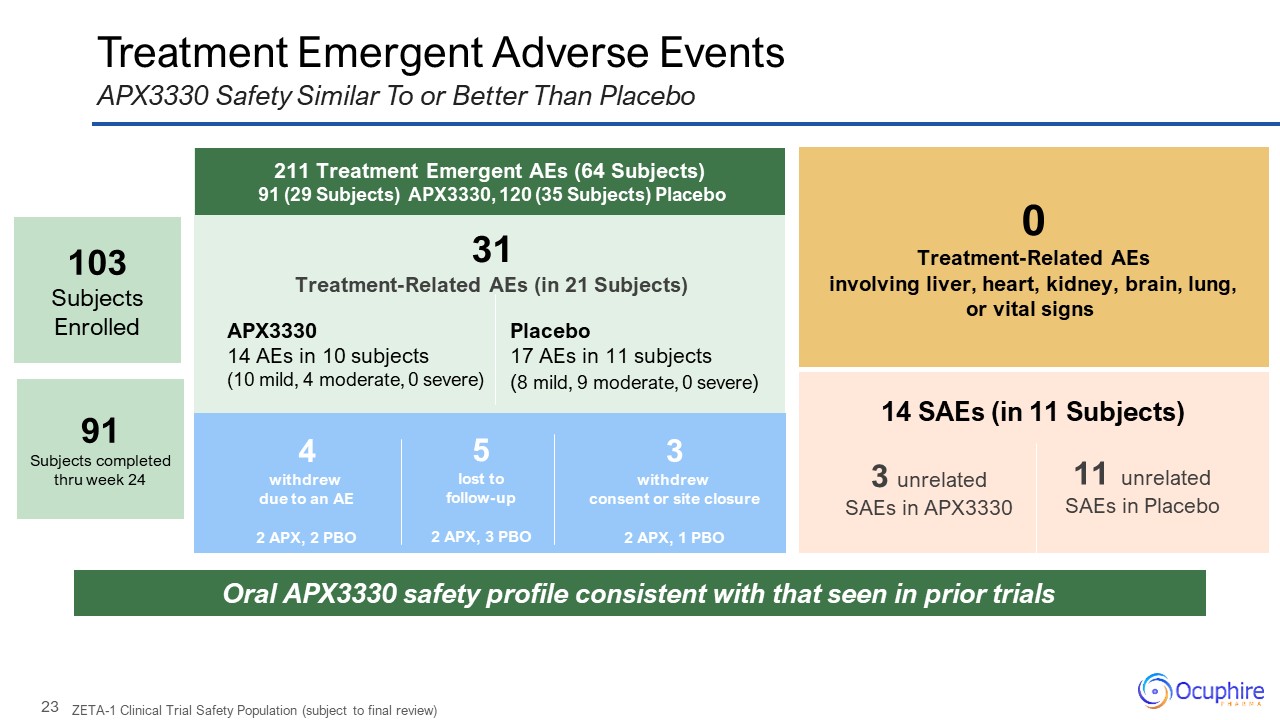
Treatment Emergent Adverse Events APX3330 Safety Similar To or Better Than
Placebo 0 Treatment-Related AEs involving liver, heart, kidney, brain, lung, or vital signs Oral APX3330 safety profile consistent with that seen in prior trials 14 SAEs (in 11 Subjects) 3 unrelated SAEs in APX3330 11
unrelated SAEs in Placebo 211 Treatment Emergent AEs (64 Subjects) 91 (29 Subjects) APX3330, 120 (35 Subjects) Placebo 31 Treatment-Related AEs (in 21 Subjects) APX3330 14 AEs in 10 subjects (10 mild, 4 moderate, 0
severe) 4 withdrew due to an AE 2 APX, 2 PBO 3 withdrew consent or site closure 2 APX, 1 PBO 5 lost to follow-up 2 APX, 3 PBO ZETA-1 Clinical Trial Safety Population (subject to final review) Placebo 17 AEs in 11 subjects
(8 mild, 9 moderate, 0 severe) 103 Subjects Enrolled 91Subjects completed thru week 24

Summary and Next Steps
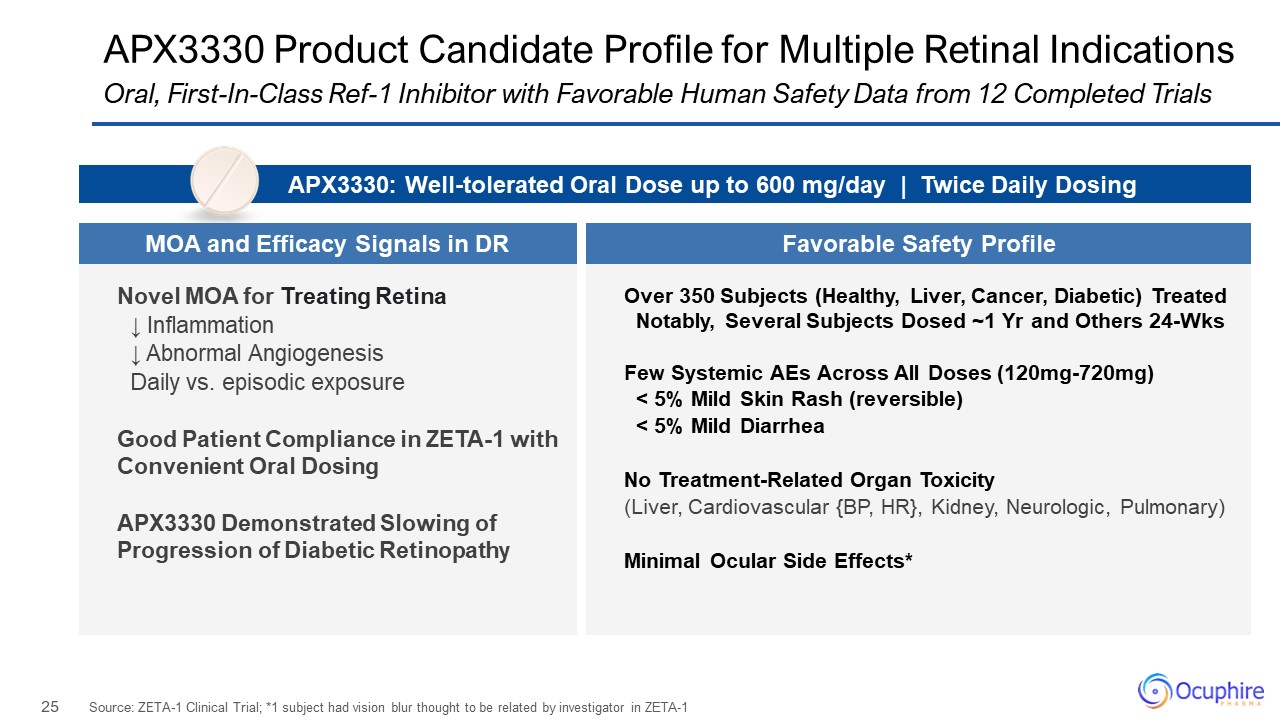
APX3330 Product Candidate Profile for Multiple Retinal Indications Source:
ZETA-1 Clinical Trial; *1 subject had vision blur thought to be related by investigator in ZETA-1 Oral, First-In-Class Ref-1 Inhibitor with Favorable Human Safety Data from 12 Completed Trials Novel MOA for Treating Retina ↓
Inflammation ↓ Abnormal Angiogenesis Daily vs. episodic exposure Good Patient Compliance in ZETA-1 with Convenient Oral Dosing APX3330 Demonstrated Slowing of Progression of Diabetic Retinopathy Over 350 Subjects (Healthy, Liver,
Cancer, Diabetic) Treated Notably, Several Subjects Dosed ~1 Yr and Others 24-Wks Few Systemic AEs Across All Doses (120mg-720mg) < 5% Mild Skin Rash (reversible) < 5% Mild Diarrhea No Treatment-Related Organ Toxicity (Liver,
Cardiovascular {BP, HR}, Kidney, Neurologic, Pulmonary) Minimal Ocular Side Effects* APX3330: Well-tolerated Oral Dose up to 600 mg/day | Twice Daily Dosing MOA and Efficacy Signals in DR Favorable Safety Profile
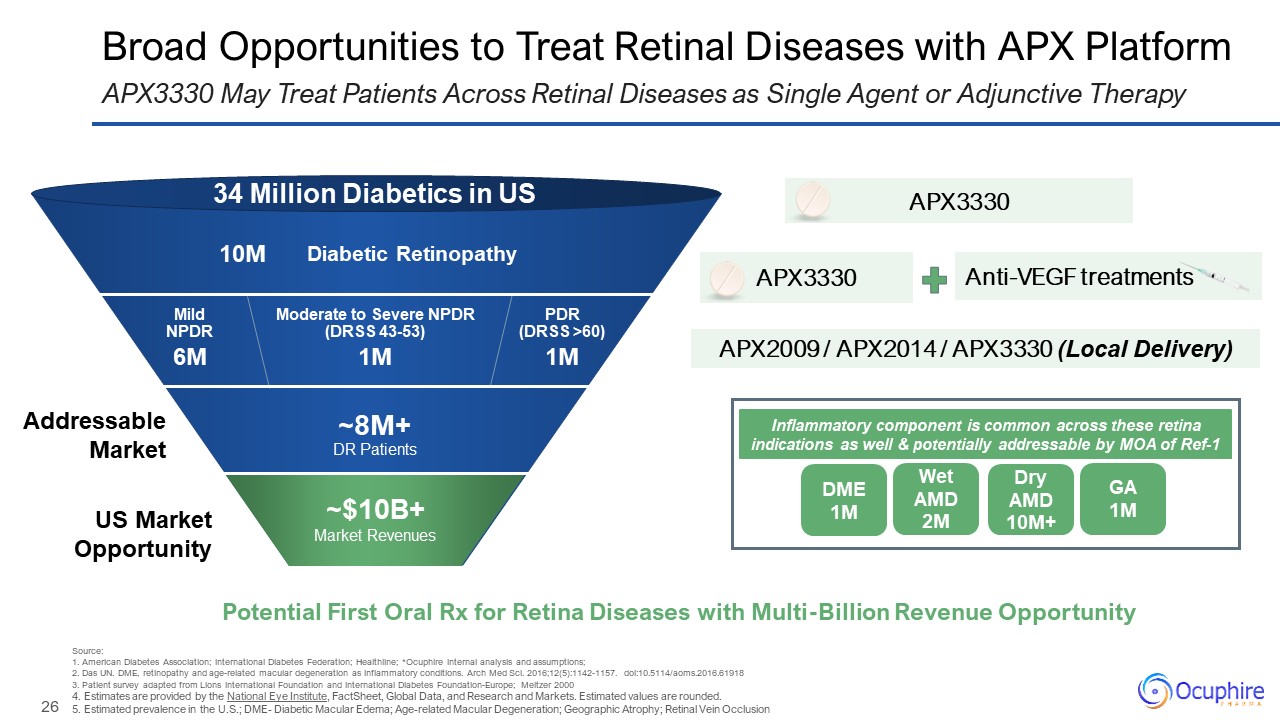
Diabetic Retinopathy PDR(DRSS >60) Moderate to Severe NPDR (DRSS
43-53) Mild NPDR 34 Million Diabetics in US 10M 6M 1M 1M ~8M+DR Patients Broad Opportunities to Treat Retinal Diseases with APX Platform APX3330 May Treat Patients Across Retinal Diseases as Single Agent or Adjunctive
Therapy Anti-VEGF treatments APX3330 APX2009 / APX2014 / APX3330 (Local Delivery) US Market Opportunity Addressable Market Potential First Oral Rx for Retina Diseases with Multi-Billion Revenue Opportunity APX3330 Source:1. American
Diabetes Association; International Diabetes Federation; Healthline; *Ocuphire internal analysis and assumptions; 2. Das UN. DME, retinopathy and age-related macular degeneration as inflammatory conditions. Arch Med Sci.
2016;12(5):1142-1157. doi:10.5114/aoms.2016.61918 3. Patient survey adapted from Lions International Foundation and International Diabetes Foundation-Europe; Meltzer 2000 4. Estimates are provided by the National Eye Institute, FactSheet,
Global Data, and Research and Markets. Estimated values are rounded. 5. Estimated prevalence in the U.S.; DME- Diabetic Macular Edema; Age-related Macular Degeneration; Geographic Atrophy; Retinal Vein Occlusion ~$10B+ Market
Revenues DME 1M Wet AMD 2M Dry AMD 10M+ GA 1M Inflammatory component is common across these retina indications as well & potentially addressable by MOA of Ref-1
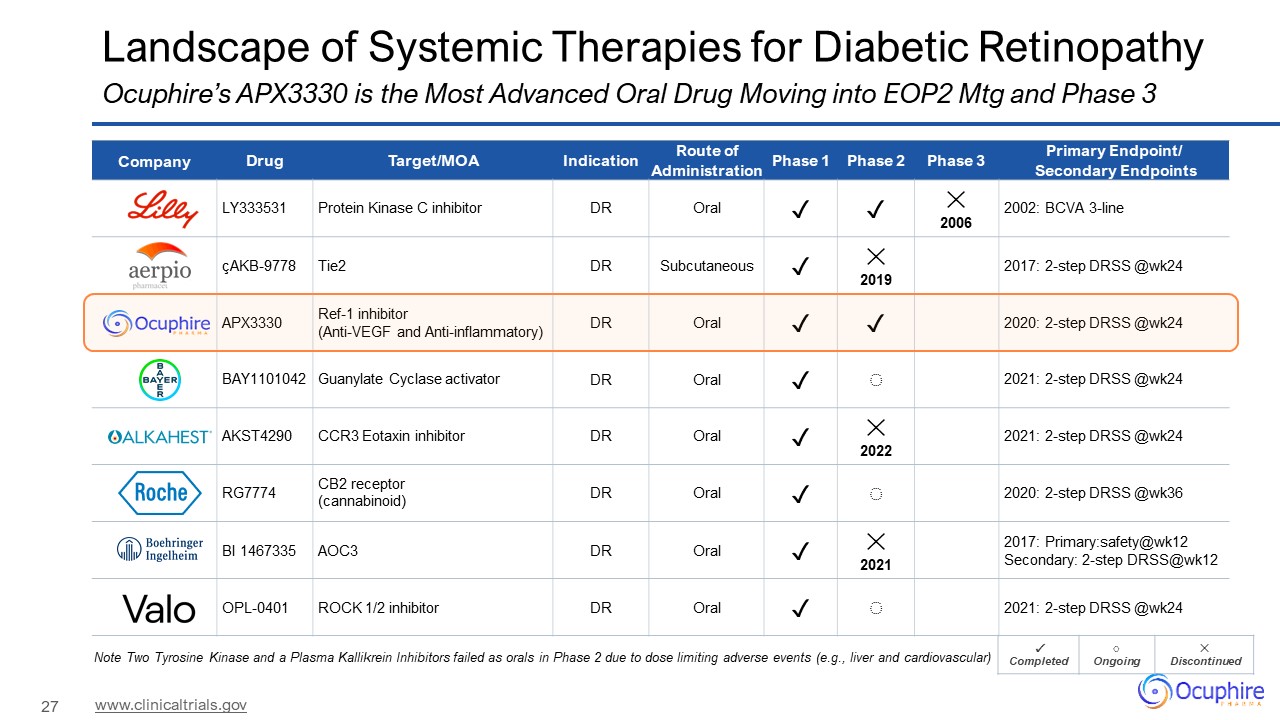
Landscape of Systemic Therapies for Diabetic Retinopathy Ocuphire’s APX3330 is
the Most Advanced Oral Drug Moving into EOP2 Mtg and Phase 3 www.clinicaltrials.gov Company Drug Target/MOA Indication Route of Administration Phase 1 Phase 2 Phase 3 Primary Endpoint/ Secondary Endpoints LY333531 Protein Kinase C
inhibitor DR Oral ✓ ✓ ✕ 2006 2002: BCVA 3-line çAKB-9778 Tie2 DR Subcutaneous ✓ ✕ 2019 2017: 2-step DRSS @wk24 APX3330 Ref-1 inhibitor (Anti-VEGF and Anti-inflammatory) DR Oral ✓ ✓ 2020: 2-step DRSS
@wk24 BAY1101042 Guanylate Cyclase activator DR Oral ✓ ◌ 2021: 2-step DRSS @wk24 AKST4290 CCR3 Eotaxin inhibitor DR Oral ✓ ✕ 2022 2021: 2-step DRSS @wk24 RG7774 CB2 receptor (cannabinoid) DR Oral ✓ ◌ 2020: 2-step DRSS
@wk36 BI 1467335 AOC3 DR Oral ✓ ✕ 2021 2017: Primary:safety@wk12 Secondary: 2-step DRSS@wk12 OPL-0401 ROCK 1/2 inhibitor DR Oral ✓ ◌ 2021: 2-step DRSS @wk24 ✓ Completed ◌ Ongoing ✕ Discontinued Note Two Tyrosine
Kinase and a Plasma Kallikrein Inhibitors failed as orals in Phase 2 due to dose limiting adverse events (e.g., liver and cardiovascular)
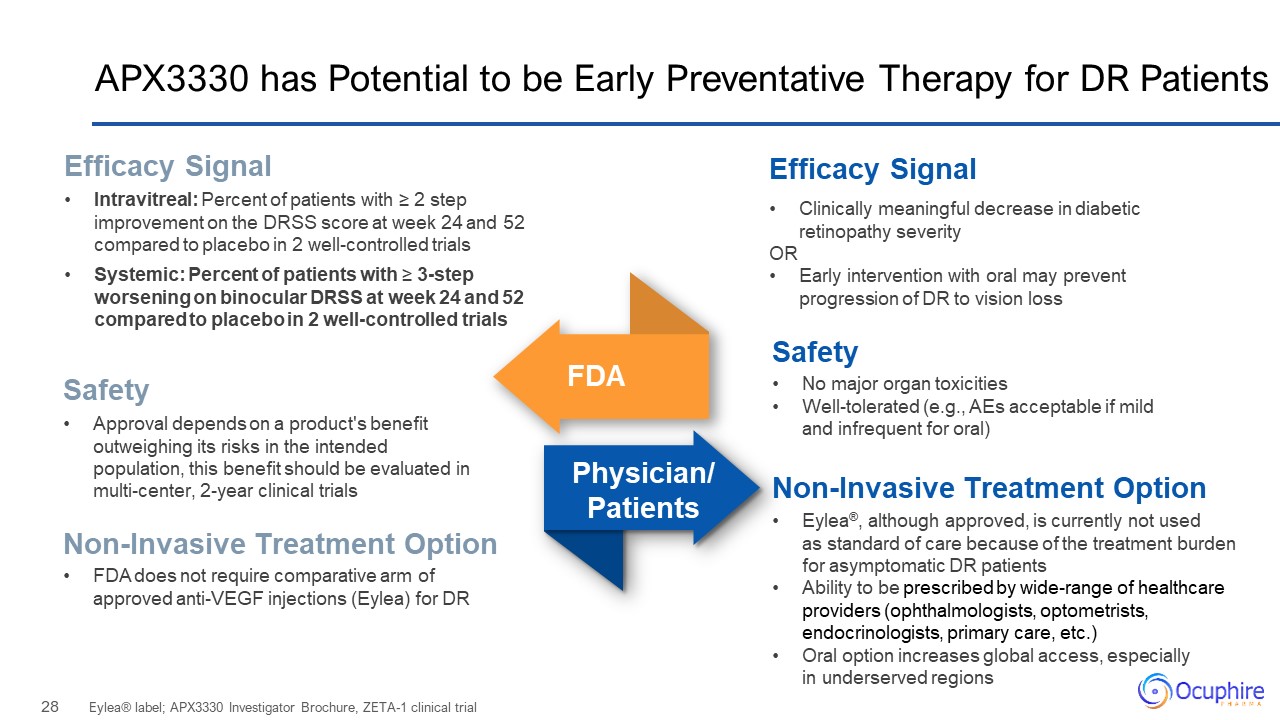
APX3330 has Potential to be Early Preventative Therapy for DR Patients Eylea®
label; APX3330 Investigator Brochure, ZETA-1 clinical trial Clinically meaningful decrease in diabetic retinopathy severity OR Early intervention with oral may prevent progression of DR to vision loss Efficacy Signal Intravitreal:
Percent of patients with ≥ 2 step improvement on the DRSS score at week 24 and 52 compared to placebo in 2 well-controlled trials Systemic: Percent of patients with ≥ 3-step worsening on binocular DRSS at week 24 and 52 compared to placebo
in 2 well-controlled trials Efficacy Signal FDA Physician/Patients Safety Non-Invasive Treatment Option Safety Approval depends on a product's benefit outweighing its risks in the intended population, this benefit should be evaluated
in multi-center, 2-year clinical trials FDA does not require comparative arm of approved anti-VEGF injections (Eylea) for DR Non-Invasive Treatment Option No major organ toxicities Well-tolerated (e.g., AEs acceptable if mild and
infrequent for oral) Eylea®, although approved, is currently not used as standard of care because of the treatment burden for asymptomatic DR patients Ability to be prescribed by wide-range of healthcare providers (ophthalmologists,
optometrists, endocrinologists, primary care, etc.) Oral option increases global access, especially in underserved regions
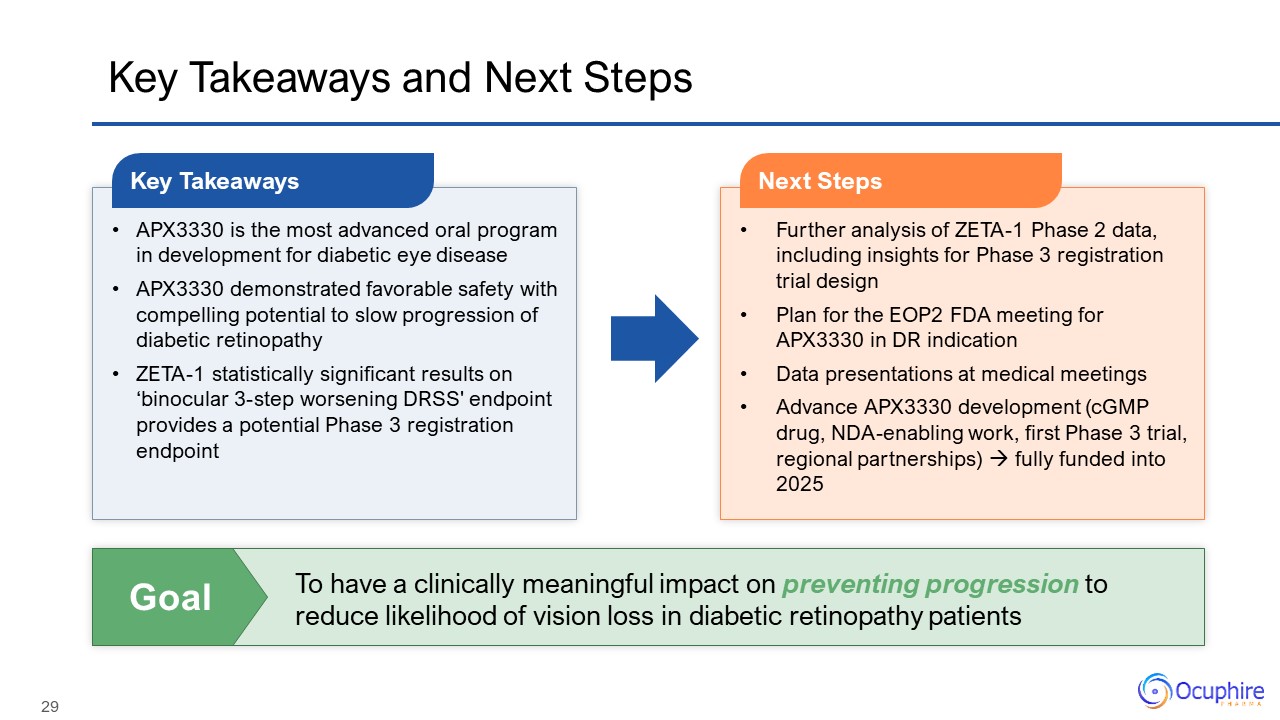
Key Takeaways and Next Steps APX3330 is the most advanced oral program in
development for diabetic eye disease APX3330 demonstrated favorable safety with compelling potential to slow progression of diabetic retinopathy ZETA-1 statistically significant results on ‘binocular 3-step worsening DRSS' endpoint provides
a potential Phase 3 registration endpoint Key Takeaways Further analysis of ZETA-1 Phase 2 data, including insights for Phase 3 registration trial design Plan for the EOP2 FDA meeting for APX3330 in DR indication Data presentations at
medical meetings Advance APX3330 development (cGMP drug, NDA-enabling work, first Phase 3 trial, regional partnerships) fully funded into 2025 Next Steps To have a clinically meaningful impact on preventing progression to reduce
likelihood of vision loss in diabetic retinopathy patients Goal

Corporate Highlights Ocuphire PharmaNasdaq: OCUP Upcoming Catalysts: Topline
Results APX3330 ZETA-1 P2b trial for DR/DME (Early 2023) EOP2 FDA Meeting for APX3330 (2H 2023) Pivotal Phase 3 Trials for Nyxol in Presbyopia with 1st Data Readouts (Late 2023) Potential Approval of 1st Nyxol NDA (Late 2023) Two Lead
Clinical-Stage Novel Drugs Addressing Multiple Large Ophthalmology Markets (~$20B US total) with Limited to No Competition & Patent Coverage to 2034+ 1 As of close on January 24, 2023; 2 End of 3Q22 (10-Q); 3 Includes upfront payment
from License Agreement NDA submitted Nov 2022 for Nyxol’s first indication in RM Global License Agreement Signed in Late 2022 with Viatris to Develop and Commercialize Nyxol for All Indications in the US and Globally Successful
Execution of 5 Trials in Last 2 Years with 6 Positive Phase 3 & Phase 2 Data Read-outs for Nyxol in RM, Presbyopia, and NVD Strong Financial Position (with No Debt) to Support Operations into 2025 and Coverage from 5 Biotech Research
Analysts Stock Price1 $3.67 Market Cap1 $77M Cash (Pro-Forma)2,3 ~$49 M Shares Outstanding2 20.8M Average Daily Volume ~200k Shares Cash Runway Into 2025 APX3330 oral tablets Diabetic Retinopathy/Diabetic Macular Edema (DR/DME) –
diabetic eye disease Nyxol preservative-free eyedrops Reversal of Mydriasis (RM) – eye dilation Presbyopia (P) – age-related blurry near vision Night Vision Disturbances (NVD) – halos, glares, starbursts

www.ocuphire.com [email protected] Ocuphire Pharma Thank You and Q&A