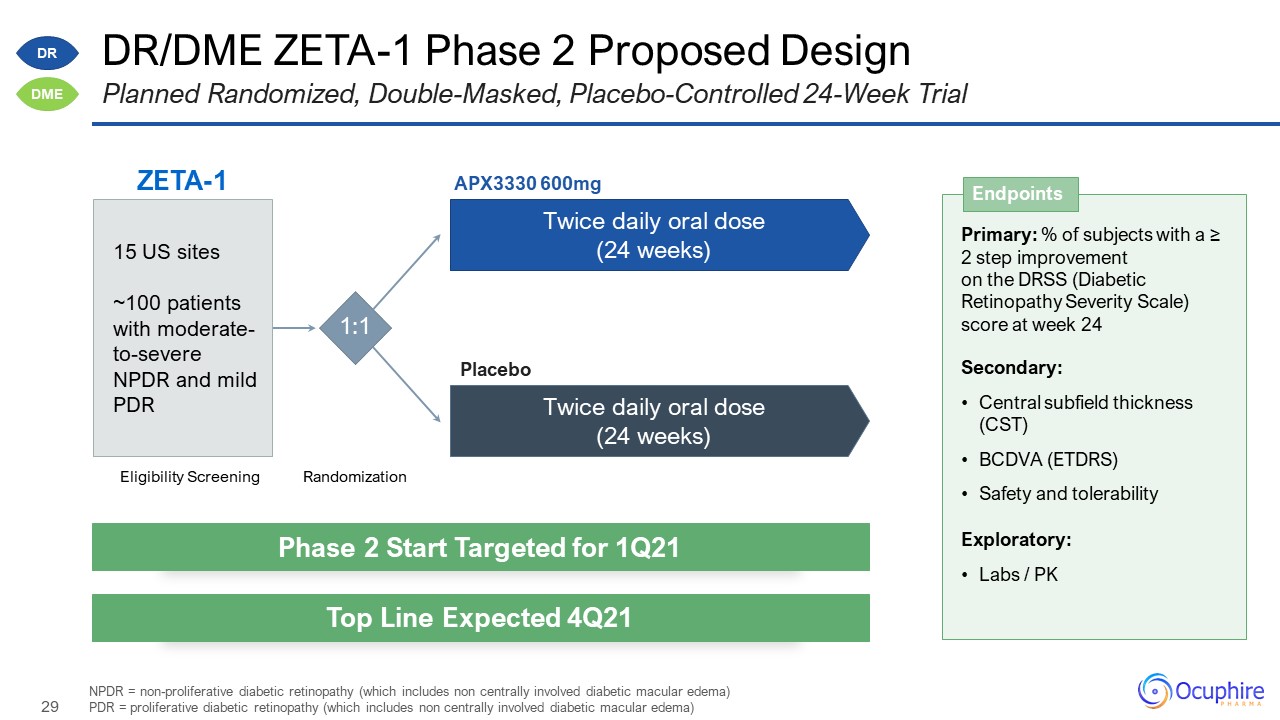
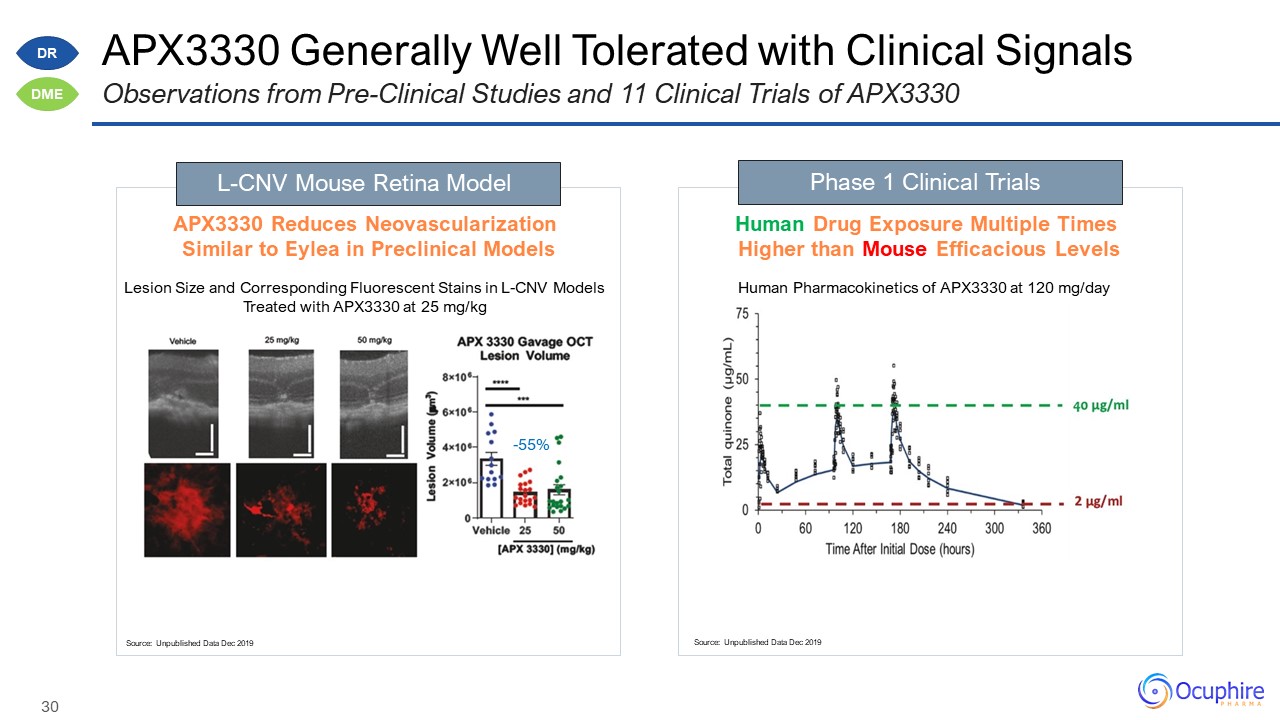
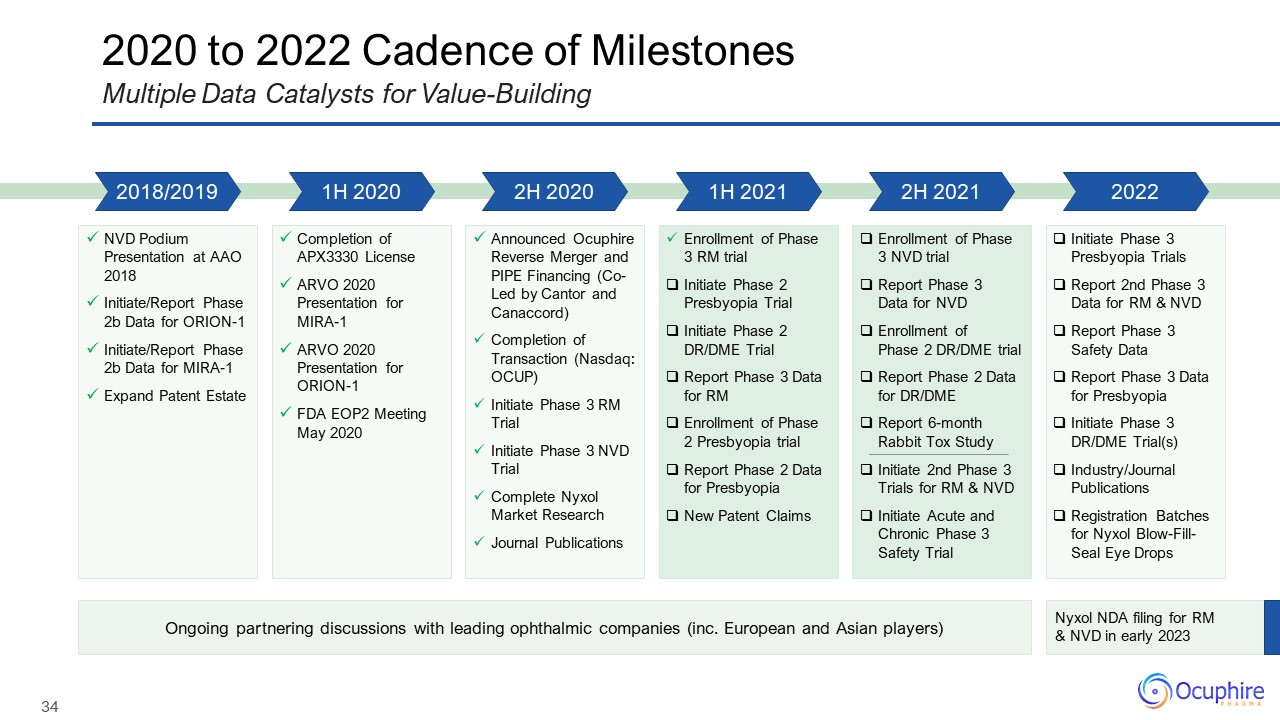
Disclosures and Forward Looking Statements This presentation contains “forward-looking
statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements concerning Ocuphire Pharma, Inc.’s (“Ocuphire” or the “Company”) product candidates and
potential. These forward-looking statements are based upon the Company’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from
those anticipated in such forward-looking statements as a result of various risks and uncertainties, including, without limitation: (i) potential adverse reactions or changes to business relationships resulting from the announcement or completion
of the merger; (ii) the success and timing of regulatory submissions and pre-clinical and clinical trials; (iii) regulatory requirements or developments; (iv) changes to clinical trial designs and regulatory pathways; (v) changes in capital
resource requirements; (vi) risks related to the inability of the Company to obtain sufficient additional capital to continue to advance its product candidates and its preclinical programs; (vii) legislative, regulatory, political and economic
developments, and (viii) the effects of COVID-19 on clinical programs and business operations. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should
be read in conjunction with statements that are included herein and elsewhere, including the risk factors detailed in documents that have been and may be filed by the Company from time to time with the SEC. All forward-looking statements
contained in this presentation speak only as of the date on which they were made. The Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. The
Company makes no representation or warranty, express or implied, as to the accuracy or completeness of the information contained in or incorporated by reference into this presentation. Nothing contained in or incorporated by reference into this
presentation is, or shall be relied upon as, a promise or representation by the Company as to the past or future. The Company assumes no responsibility for the accuracy or completeness of any such information. This presentation may not be
reproduced or provided to any other person (other than your advisor) without our prior written consent. By accepting delivery of this presentation, you agree to the foregoing and agree to return this presentation and any documents related
thereto and any copies thereof to us or to destroy the same if you do not make an investment in any securities. The information contain within this presentation shall not, except as hereinafter provided, without the prior written consent of the
Company, be disclosed by you or your representatives in any manner whatsoever, in whole or in part, and shall not be used by you or your representatives other than for the purpose of evaluating the transaction described herein. By accepting
delivery of this presentation you further acknowledge and agree aware of the restrictions imposed by the United States securities laws on the purchase or sale of securities by any person who has received material, nonpublic information from the
issuer of the securities or any affiliate thereof and on the communication of such information to any other person when it is reasonably foreseeable that such other person is likely to purchase or sell such securities in reliance on such
information for so long as the information remains material and non-public. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market shares and other data about our industry.
This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such
use should not be construed as an endorsement of such products. November 6, 2020