 | | | PROPOSED MERGER YOUR VOTE IS VERY IMPORTANT | | |  |
Douglas J. Swirsky | | | Mina Sooch |
President and CEO, Rexahn Pharmaceuticals, Inc. | | | President and CEO, Ocuphire Pharma, Inc. |
Delaware (State or other jurisdiction of incorporation or organization) | | | 2834 (Primary Standard Industrial Classification Code Number) | | | 11-3516358 (I.R.S. Employer Identification Number) |
Asher M. Rubin William I. Intner Hogan Lovells US LLP 100 International Drive, Suite 2000 Baltimore, MD 21202 (410) 659-2700 | | | Mina Sooch President & Chief Executive Officer Ocuphire Pharma, Inc. 37000 Grand River Ave, Suite 120 Farmington Hills, MI 48335 (248) 681-9815 | | | Phillip D. Torrence Jeffrey H. Kuras Emily J. Johns Honigman LLP 650 Trade Centre Way, Suite 200 Kalamazoo, MI 49002-0402 (269) 337-7700 |
Large accelerated filer | | | ☐ | | | | | Accelerated filer | | | ☐ | |
Non-accelerated filer | | | ☒ | | | | | Smaller reporting company | | | ☒ | |
| | | | | | | Emerging growth company | | | ☐ |
Title of Each Class of Security to be Registered | | | Amount to be Registered(1) | | | Proposed Maximum Offering Price Per Share | | | Proposed Maximum Aggregate Offering Price(2) | | | Amount of Registration Fee(3) |
Common stock, $0.0001 par value per share | | | 75,043,772 | | | N/A | | | $336.95 | | | $1.00 |
(1) | Represents the maximum number of shares of common stock, $0.0001 par value per share (“Rexahn common stock”), of Rexahn Pharmaceuticals, Inc., a Delaware corporation (“Rexahn”), issuable to holders of common stock, $0.0001 par value per share (“Ocuphire common stock”), and options of Ocuphire Pharma, Inc., a Delaware corporation (“Ocuphire”), in the proposed merger of Razor Merger Sub, Inc., a Delaware corporation and a wholly owned subsidiary of Rexahn (“Merger Sub”), with and into Ocuphire (the “merger”). The amount of Rexahn common stock to be registered is based on the estimated maximum number of shares of Rexahn common stock that are expected to be issued pursuant to the merger, after taking into account the expected issuance by Ocuphire immediately prior to the merger of an estimated 4,462,544 shares of Ocuphire common stock (3,346,908 of which will be held in escrow for the benefit of certain accredited investors) pursuant to an amended and restated securities purchase agreement, dated June 29, 2020, by and among Ocuphire, Rexahn and certain accredited investors, and assuming an exchange ratio calculated by assuming a minimum “Parent Valuation” of $12.0 million and “Parent Outstanding Shares” of 5,019,141 (which amount includes shares of Rexahn common stock that may be issued by Rexahn in exchange for warrants of Rexahn that are currently outstanding), conversion of Ocuphire convertible notes on November 14, 2020, and without giving effect to a reverse stock split of Rexahn common stock expected to be completed immediately prior to the merger. |
(2) | Estimated solely for purposes of calculating the registration fee in accordance with Rule 457(f)(2) of the Securities Act of 1933, as amended (the “Securities Act”). Ocuphire is a private company, no market exists for its securities, and Ocuphire has an accumulated capital deficit. Therefore, the proposed maximum aggregate offering price is one-third of the aggregate par value of the Ocuphire securities expected to be exchanged in the proposed merger. |
(3) | This fee has been calculated pursuant to Section 6(b) of the Securities Act and has been rounded up to $1.00. Rexahn previously paid this amount. |
 | | | PROPOSED MERGER YOUR VOTE IS VERY IMPORTANT | | |  |
Douglas J. Swirsky | | | Mina Sooch |
President and CEO, Rexahn Pharmaceuticals, Inc. | | | President and CEO, Ocuphire Pharma, Inc. |

1. | to consider and vote upon a proposal to approve the issuance of shares of Rexahn common stock, $0.0001 par value per share (“Rexahn common stock”), to stockholders of Ocuphire pursuant to the terms of the Agreement and Plan of Merger and Reorganization, dated as of June 17, 2020, by and among Rexahn, Merger Sub and Ocuphire, as amended by the First Amendment to Agreement and Plan of Merger and Reorganization dated June 29, 2020, a copy of which is attached as Annex A to this proxy statement/prospectus/information statement (as amended, the “Merger Agreement”), and the change of control of Rexahn resulting from the merger under The Nasdaq Stock Market LLC rules; |
2. | to consider and vote upon an amendment to the amended and restated certificate of incorporation of Rexahn, as amended (the “Rexahn Certificate of Incorporation”), to effect a reverse stock split of Rexahn common stock, at a ratio within the range of 1-for-3 to 1-for-5, with such specific ratio to be approved by the Rexahn Board, in the form attached as Annex B to this proxy statement/prospectus/information statement; |
3. | to consider and vote upon an amendment to the Rexahn Certificate of Incorporation to change the corporate name of Rexahn from “Rexahn Pharmaceuticals, Inc.” to “Ocuphire Pharma, Inc.”, in the form attached as Annex C to this proxy statement/prospectus/information statement; |
4. | to consider and vote upon a proposal to approve the adoption of the Ocuphire Pharma, Inc. 2020 Equity Incentive Plan in the form attached as Annex D to this proxy statement/prospectus/information statement (the “Ocuphire 2020 Plan”); |
5. | to consider and vote upon a proposal to approve the issuance of: (i) shares of Rexahn common stock upon the exercise of the Investor Warrants to be issued in the Pre-Merger Financing, and (ii) additional shares of Rexahn common stock that may be issued following the closing of the Pre-Merger Financing, in each case pursuant to the Amended and Restated Securities Purchase Agreement, dated as of June 29, 2020, by and among Rexahn, Ocuphire and the investors party thereto, and as required by and in accordance with Nasdaq Listing Rule 5635; |
6. | to consider and vote upon an adjournment of the Rexahn special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of Proposal No. 1, 2, 3, 4 or 5; and |
7. | to transact such other business as may properly come before the Rexahn special meeting or any adjournment or postponement thereof. |
| | | By Order of the Rexahn Board, | |
| | | ||
| | | Douglas J. Swirsky President and Chief Executive Officer Rockville, Maryland , 2020 |
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | |
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | |
Q: | What is the merger? |
A: | Rexahn, Merger Sub and Ocuphire entered into the Agreement and Plan of Merger and Reorganization on June 17, 2020 (the “Original Merger Agreement”). On June 29, 2020, the parties entered into the First Amendment to Agreement and Plan of Merger and Reorganization (the “Merger Agreement Amendment,” and together with the Original Merger Agreement, the “Merger Agreement”). The Merger Agreement contains the terms and conditions of the proposed business combination of Rexahn and Ocuphire. Under the Merger Agreement, Merger Sub will merge with and into Ocuphire, with Ocuphire surviving as a wholly owned subsidiary of Rexahn (the “merger”). |
Q: | What is the Pre-Merger Financing? |
A: | Concurrently with signing the Original Merger Agreement, Ocuphire and Rexahn entered into a securities purchase agreement with certain institutional healthcare investors, accredited investors and certain directors and officers of Ocuphire (the “Investors”). On June 29, 2020, concurrently with the execution of the Merger Agreement Amendment, Ocuphire and Rexahn entered into an amended and restated securities purchase agreement (as may be amended from time to time, the “Securities Purchase Agreement”) with the Investors, pursuant to which, among other things, (i) Ocuphire agreed to issue to the Investors shares of Ocuphire common stock (the “Initial Shares”) and to issue to an escrow account for the benefit of the Investors three times the number of Initial Shares of Ocuphire common stock (the “Additional Shares” and together with the Initial Shares, the “Pre-Merger Financing Shares”), in each case immediately prior to the merger to be exchanged for shares of Rexahn common stock at the closing of the merger, and (ii) Rexahn agreed to issue to the Investors warrants to purchase shares of Rexahn common stock on the tenth trading day following the consummation of the merger (the “warrant closing date”) (the “Investor Warrants”), and subject to certain conditions set forth in the Securities Purchase Agreement, to issue to the Investors all or a portion of the shares of Rexahn common stock from the escrow account, in a private placement transaction for an aggregate purchase price of approximately $21,150,000 (the “Pre-Merger Financing”). |
Q: | What will Ocuphire securityholders receive in the merger? |
A: | The Exchange Ratio at the closing of the merger (the “Closing”) would be approximately 4.3812, assuming (i) the Ocuphire convertible notes converted on September 10, 2020, (ii) the Parent Cash Amount is $1.9 million on the Anticipated Closing Date, (iii) there are 4,483,198 shares of Rexahn common stock outstanding as of the Closing (on a pre-Rexahn Reverse Stock Split basis) and (iv) there are 6,806,019 shares of Ocuphire common stock and options exercisable for Ocuphire common stock (each, an “Ocuphire Option,” each holder of an Ocuphire Option an “Ocuphire Optionholder” and, collectively with the Ocuphire Stockholders, “Ocuphire Securityholders”) outstanding as of the Closing (giving effect to the Initial Shares issued in the Pre-Merger Financing, and shares issuable upon the conversion of the Ocuphire convertible notes). |
Q: | What will Rexahn Securityholders receive in the merger? |
A: | At the Effective Time, Rexahn Stockholders will continue to own and hold their existing shares of Rexahn common stock. |
Q: | What will happen to Rexahn if, for any reason, the merger does not close? |
A: | If, for any reason, the merger does not close, the Rexahn Board may elect to, among other things, attempt to complete another strategic transaction like the merger, attempt to sell or otherwise dispose of the various assets of Rexahn, resume its research and development activities and continue to operate the business of Rexahn or dissolve and liquidate its assets. If Rexahn decides to dissolve and liquidate its assets, Rexahn would be required to pay all of its debts and contractual obligations, and to set aside certain reserves for potential future claims, and there can be no assurances as to the amount or timing of available cash left, if any, to distribute to stockholders after paying the debts and other obligations of Rexahn and setting aside funds for reserves. If Rexahn were to continue its business, it would need to raise a substantial amount of cash to fund ongoing operations and future development activities for its existing product candidates and any new product candidates that it acquires. |
Q: | Why are the two companies proposing to merge? |
A: | Ocuphire and Rexahn believe that the merger will result in a clinical-stage ophthalmic biopharmaceutical company focused on developing and commercializing therapies for the treatment of eye disorders. For a discussion of Rexahn’s and Ocuphire’s reasons for the merger, please see the section entitled “The Merger—Rexahn Reasons for the Merger” and “The Merger—Ocuphire Reasons for the Merger” in this proxy statement/prospectus/information statement. |
Q: | Why am I receiving this proxy statement/prospectus/information statement? |
A: | You are receiving this proxy statement/prospectus/information statement because you have been identified as a Rexahn Stockholder or an Ocuphire Stockholder as of the applicable record date, and you are entitled, as applicable, to (i) notice of, and to vote at, the Rexahn special meeting or (ii) sign and return to Ocuphire the written consent. This document serves as: |
• | a proxy statement of Rexahn used to solicit proxies for the Rexahn special meeting; |
• | a prospectus of Rexahn used to offer shares of Rexahn common stock in exchange for shares of Ocuphire common stock in the merger and issuable upon exercise of Ocuphire Options; and |
• | an information statement of Ocuphire used to solicit the written consent of Ocuphire Stockholders for the adoption of the Merger Agreement and the approval of the merger and related transactions. |
Q: | What is required to consummate the merger? |
A: | To consummate the merger, Rexahn Stockholders must approve (i) the issuance of Rexahn common stock to Ocuphire Stockholders pursuant to the Merger Agreement and the change of control of Rexahn resulting from the merger under Nasdaq rules (Proposal No. 1) and (ii) the Rexahn Reverse Stock Split (Proposal No. 2). The merger may also not be consummated if Proposal Nos. 3 or 5 are not approved as approval of such proposals is also a condition to Closing under the Merger Agreement. Ocuphire Stockholders must adopt the Merger Agreement, thereby approving the merger and the related transactions. |
Q: | What stockholder votes are required to approve the proposals required in connection with the merger at the Rexahn special meeting? |
Q: | Who will be the directors of Rexahn following the merger? |
A: | Following the consummation of the merger, the size of the Rexahn Board is expected to be comprised of seven directors. Pursuant to the terms of the Merger Agreement, the Rexahn Board will be reconstituted such that six of the initial post-Closing directors will be designated by Ocuphire, and one initial post-Closing director will be designated by Rexahn. It is currently anticipated that, following the Closing, the Rexahn Board will be constituted as follows: |
Name | | | Current Principal Affiliation |
Mina Sooch | | | Ocuphire Pharma, Inc., President, Chief Executive Officer and Director |
Sean Ainsworth | | | Ocuphire Pharma, Inc., Director |
Alan R. Meyer | | | Ocuphire Pharma, Inc., Director |
James S. Manuso | | | Ocuphire Pharma, Inc., Director |
Cam Gallagher | | | Ocuphire Pharma, Inc., Director |
Richard J. Rodgers | | | Rexahn Pharmaceuticals, Inc., Director |
Susan K. Benton | | | Thea Pharma, Inc., General Manager and Head of the U.S. |
Q: | Who will be the executive officers of Rexahn immediately following the merger? |
A: | Immediately following the consummation of the merger, the executive management team of Rexahn is expected to be composed solely of the members of Ocuphire’s executive management team prior to the merger, as follows: |
Name | | | Title |
Mina Sooch, MBA | | | President, Chief Executive Officer & Treasurer |
Bernhard Hoffmann, MBA | | | VP of Corporate Development & Finance, Secretary |
Q: | What are the material U.S. federal income tax consequences of the merger? |
A: | In the opinion of Honigman LLP (“Honigman”), counsel to Ocuphire, and subject to the Tax Opinion Representations and Assumptions (as defined on page 158), the merger will qualify as a “reorganization” within the meaning of Section 368(a) of the Internal Revenue Code of 1986, as amended (the “Code”). Subject to the limitations and qualifications described in the section entitled “The Merger—Material U.S. Federal Income Tax Consequences of the Merger,” a U.S. Holder (as defined on page 157) of Ocuphire common stock will not recognize any gain or loss for U.S. federal income tax purposes on the exchange of shares of Ocuphire common stock for shares of Rexahn common stock in the merger, except with respect to cash received by such U.S. Holder of Ocuphire common stock in lieu of a fractional share of Rexahn common stock. If any of the Tax Opinion Representations and Assumptions is incorrect, incomplete or inaccurate or is violated, the accuracy of the opinion described above may be affected and the U.S. federal income tax consequences of the merger could differ from those described in this proxy statement/prospectus/information statement. |
Q: | What are the material U.S. federal income tax consequences of the receipt of CVRs and the Rexahn Reverse Stock Split to Rexahn U.S. Holders? |
A: | In the opinion of Hogan Lovells US LLP, Rexahn’s legal counsel, based on the facts, representations and assumptions set forth herein, the issuance of the CVRs to Rexahn U.S. Holders (as defined on page 185) under the terms expressed in the form of the CVR Agreement included in Annex G to this proxy statement/prospectus/information statement is more likely than not to be treated as a distribution of property with respect to Rexahn common stock. Please review the information in the section entitled “Agreements Related to the Merger—Contingent Value Rights Agreement—Material U.S. Federal Income Tax Consequences of the Receipt of CVRs” for a more complete description of the material U.S. federal income tax consequences of the receipt of CVRs to Rexahn U.S. Holders, including possible alternative treatments. |
Q: | As a Rexahn Stockholder, how does the Rexahn Board recommend that I vote? |
A: | After careful consideration, the Rexahn Board recommends that Rexahn Stockholders vote “FOR” all of the proposals described in this proxy statement/prospectus/information statement. |
Q: | As an Ocuphire Stockholder, how does the Ocuphire Board recommend that I vote? |
A: | After careful consideration, the Ocuphire Board recommends that Ocuphire Stockholders execute the written consent to approve a certificate of amendment to Ocuphire’s certificate of incorporation, as amended (the “Ocuphire Certificate of Incorporation”) to increase the authorized shares of Ocuphire common stock, the merger, the Merger Agreement, and the transactions contemplated therein, substantially in accordance with the terms of the Merger Agreement and the other agreements contemplated by the Merger Agreement. |
Q: | What risks should I consider in deciding whether to vote in favor of the merger or to execute and return the written consent, as applicable? |
A: | You should carefully review the section entitled “Risk Factors” in this proxy statement/prospectus/information statement which sets forth certain risks and uncertainties related to the merger, risks and uncertainties to which the combined company’s business will be subject, and risks and uncertainties to which each of Rexahn and Ocuphire, as independent companies, are subject. |
Q: | Who can vote at the Rexahn special meeting? |
A: | Only Rexahn Stockholders of record at the close of business on the Record Date will be entitled to vote at the Rexahn special meeting. As of the Record Date, there were 4,483,198 shares of Rexahn common stock outstanding and entitled to vote. |
Q: | How many votes do I have? |
A: | On each matter to be voted upon, you have one vote for each share of Rexahn common stock you own as of the Record Date. |
Q: | What is the quorum requirement? |
A: | A quorum of Rexahn Stockholders is necessary to hold a valid meeting. A quorum will be present if Rexahn Stockholders holding at least 40% of the issued and outstanding shares of Rexahn common stock entitled to vote at the Rexahn special meeting are present in person or represented by proxy at the Rexahn special meeting. As of the Record Date, there were 4,483,198 shares of Rexahn common stock outstanding and entitled to vote. Accordingly, Rexahn expects that the holders of at least 1,793,280 shares of Rexahn common stock must be present at the Rexahn special meeting for a quorum to exist. Your shares of Rexahn common stock will be counted toward the quorum at the Rexahn special meeting only if you attend the Rexahn special meeting in person or are represented at the Rexahn special meeting by proxy. |
Q: | What are “broker non-votes?” |
A: | If you hold shares beneficially in street name and do not provide your broker or other agent with voting instructions, your shares may constitute “broker non-votes.” Broker non-votes occur on a matter when banks, brokers and other nominees are not permitted to vote on certain non-discretionary matters without instructions from the beneficial owner and instructions are not given. These matters are referred to as “non-routine” matters. Proposal Nos. 1, 4 and 5 are anticipated to be non-routine matters, and Proposal Nos. 2, 3, and 6 are anticipated to be routine matters. Broker non-votes will have no effect on the outcome of Proposal Nos. 1, 4, and 5. |
Q: | When do you expect the merger to be consummated? |
A: | Rexahn and Ocuphire anticipate that the merger will occur sometime soon after the Rexahn special meeting to be held on Monday, November 2, 2020, but the companies cannot predict the exact timing. For more information, please see the section entitled “The Merger Agreement—Conditions to the Completion of the Merger” in this proxy statement/prospectus/information statement. |
Q: | What do I need to do now? |
A: | Rexahn and Ocuphire urge you to read this proxy statement/prospectus/information statement carefully, including its annexes, and to consider how the merger affects you. |
Q: | What happens if I do not return a proxy card or otherwise provide proxy instructions, as applicable? |
A: | If you are a Rexahn Stockholder of record, the failure to return your proxy card or otherwise provide proxy instructions will have the same effect as voting “AGAINST” Proposal Nos. 2 and 3. |
Q: | When and where is the Rexahn special meeting and may I vote in person? |
A: | The Rexahn special meeting will be held at Rexahn’s offices located at 15245 Shady Grove Road, Suite 455, Rockville, MD 20850, at 8:00 a.m., Eastern Time, on Monday, November 2, 2020. Subject to space availability, all Rexahn Stockholders as of the Record Date, or their duly appointed proxies, may attend the Rexahn special meeting. Since seating is limited, admission to the Rexahn special meeting will be on a first-come, first-served basis. Registration and seating will begin at 7:30 a.m., Eastern Time. If your shares of Rexahn common stock are registered directly in your name with Rexahn’s transfer agent, you are considered to be the stockholder of record with respect to those shares, and the proxy materials and proxy card are being sent directly to you by Rexahn. If you are a stockholder of record, you may attend the Rexahn special meeting and vote your shares in person. Even if you plan to attend the Rexahn special meeting in person, Rexahn requests that you sign and return the enclosed proxy to ensure that your shares will be represented at the Rexahn special meeting if you become unable to attend. If your shares of Rexahn common stock are held in a brokerage account or by another nominee, you are considered the beneficial owner of shares held in “street name,” and the proxy materials are being forwarded to you by your broker or other nominee together with a voting instruction card. As the beneficial owner, you are also invited to attend the Rexahn special meeting. Because a beneficial owner is not the stockholder of record, you may not vote these shares in person at the Rexahn special meeting unless you obtain a proxy from the broker, trustee or nominee that holds your shares, giving you the right to vote the shares at the Rexahn special meeting. |
Q: | If my Rexahn shares are held in “street name” by my broker, will my broker vote my shares for me? |
A: | Unless your broker has discretionary authority to vote on certain matters, your broker will not be able to vote your shares of Rexahn common stock without instructions from you. Brokers are not expected to have discretionary authority to vote for any of the proposals other than Proposal Nos. 2, 3, and 6. To make sure that your vote is counted, you should instruct your broker to vote your shares, following the procedures provided by your broker. |
Q: | May I change my vote after I have submitted a proxy or provided proxy instructions? |
A: | Rexahn Stockholders of record may change their vote at any time before their proxy is voted at the Rexahn special meeting in one of three ways. First, a Rexahn Stockholder of record can send a written notice to the Secretary of Rexahn stating that it would like to revoke its proxy. Second, a Rexahn Stockholder of record can submit new proxy instructions either on a new proxy card or via telephone or the Internet. Third, a Rexahn Stockholder of record can attend the Rexahn special meeting and vote in person. Attendance alone will not revoke a proxy. If a Rexahn Stockholder who owns shares of Rexahn common stock in “street name” has instructed a broker to vote its shares of Rexahn common stock, the stockholder must follow directions received from its broker to change those instructions. |
Q: | Who is paying for this proxy solicitation? |
A: | Rexahn and Ocuphire will share equally the cost of printing and filing this proxy statement/prospectus/information statement and the proxy card. Arrangements will also be made with brokerage firms and other custodians, nominees and fiduciaries who are record holders of Rexahn common stock for the forwarding of solicitation materials to the beneficial owners of Rexahn common stock. Rexahn will reimburse these brokers, custodians, nominees and fiduciaries for the reasonable out-of-pocket expenses they incur in connection with the forwarding of solicitation materials. |
Q: | Who can help answer my questions? |
A: | If you are a Rexahn Stockholder and would like additional copies, without charge, of this proxy statement/prospectus/information statement or if you have questions about the merger, including the procedures for voting your shares, you should contact: |
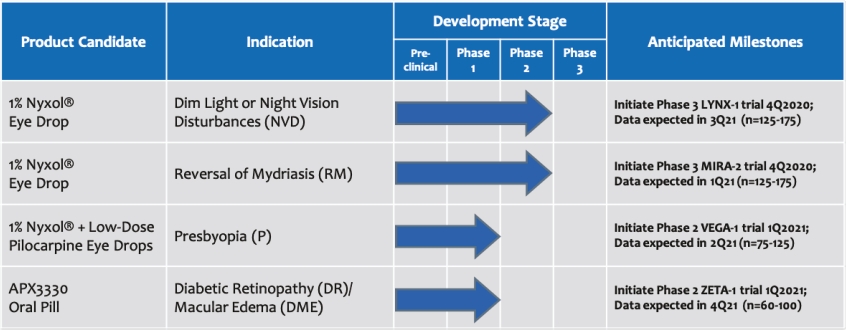
• | Lead Product Candidate Nyxol is Phase 3 Ready in Multiple Indications. Nyxol is being developed for the treatment of multiple indications, which Ocuphire management believes together represent a significant market opportunity. Ocuphire plans to begin Phase 3 trials for NVD and RM in the fourth quarter of 2020, and Phase 2 development for presbyopia in the first quarter of 2021. |
• | Secondary Product Candidate APX3330 to Initiate Phase 2 Clinical Development. APX3330 is being developed for DR and DME, which represents a significant, established market opportunity, with plans to begin its first ophthalmic Phase 2 trial in the first quarter of 2021. APX2009, a second generation preclinical product candidate analog of APX3330, is being investigated for use in wet age-related macular degeneration (“wAMD”). Wet age-related macular degeneration is a chronic and progressive disease where abnormal blood vessels grow underneath the retina and leak blood and fluid into the macula. |
• | Multiple Upcoming Late Clinical Stage Milestones. Ocuphire expects top-line results to read out as early as the first quarter of 2021 and throughout the remainder of 2021 for its four planned clinical trials. |
• | Experienced Management Team. It is expected that the combined organization will be led by the experienced senior management team from Ocuphire and a board of directors from Ocuphire with representation from Rexahn. |
• | Cash Resources. Following the Closing and taking into account proceeds received in the Pre-Merger Financing, the combined company is expected to have sufficient cash at the Closing for the combined company to sustain its operations through 2021. The combined company’s Nasdaq listing will provide it with access to the public market to raise additional funds in the future. |
• | the Rexahn Board’s belief that a go-it-alone scenario poses significant risk, including the risk of dilution to the Rexahn Stockholders, taking into account Rexahn’s business, operational and financial prospects, including its cash position, the limited value given by the marketplace to Rexahn’s product portfolio, uncertainty regarding the potential results from additional preclinical studies and clinical trials, uncertainty regarding the future costs and timeline to support a clinical program of Rexahn’s product candidates, the chances of success in conducting a clinical development program and obtaining regulatory approval, and the need to raise significant additional financing for future clinical and commercial development of Rexahn’s product candidates; |
• | the Rexahn Board’s belief, given the risks associated with clinical development, and based in part on the judgment, advice and analysis of Rexahn senior management with respect to the potential strategic, financial and operational benefits of the merger (which judgment was informed in part by the business, technical, financial and legal due diligence investigation performed by Rexahn with respect to Ocuphire) that Ocuphire’s Phase 3 ready, lead product candidate, Nyxol, for multiple front-of-the-eye (pupil/cornea) indications, as well as its product candidate, APX3330, for multiple back-of-the-eye (retina) conditions, along with the experience of its management and other personnel, and the granting of CVRs to Rexahn Stockholders to provide a potential financial benefit in the event that any of Rexahn’s existing intellectual property is sold or licensed during a future period or Rexahn receives any payments from BioSense or HaiChang, would create more value for Rexahn Stockholders in the long term than Rexahn could create as an independent stand-alone company; |
• | the Rexahn Board’s review of the current development plans of Ocuphire to confirm the likelihood that the combined company would possess sufficient resources, or have access to sufficient resources, to allow Ocuphire senior management to focus on its plans for the continued development of Ocuphire’s product pipeline; |
• | the Rexahn Board’s consideration that the combined company should have sufficient cash at the Closing for the combined company to sustain its operations for the next 18 months at the time of the Rexahn Board’s consideration and the combined company’s public company structure will provide it with access to the public market to raise additional funds in the future; |
• | the Rexahn Board’s consideration of the results of its strategic review process, which included Oppenheimer’s outreach to 50 companies and the receipt of five inbound inquiries, resulting in the receipt of indications of interest from 19 companies. Further, the Rexahn Board’s consideration of the valuation and business prospects of all other strategic transaction candidates involved in its strategic review process, and its collective view that Ocuphire was the most attractive candidate for Rexahn due to, among other things, Ocuphire’s Phase 3 ready asset, Nyxol, as well as its APX3330 product candidate, Ocuphire’s strong financial position that includes backing from a syndicate of investors, the strength of Ocuphire’s management team, the potential market opportunity for Nyxol and APX3330, Ocuphire’s understanding of the potential value of Rexahn’s partnerships with BioSense and HaiChang, and that Ocuphire’s potential to achieve key milestones over the next several years could enable the combined company to access the public markets for additional financial resources; |
• | the Rexahn Board’s conclusion that the merger provides existing Rexahn Stockholders a significant opportunity to participate in the potential growth of the combined company following the merger, while potentially receiving certain cash payments from the grant, sale or transfer of rights to Rexahn’s existing intellectual property or pursuant to payments received by BioSense or HaiChang during a certain period following Closing on account of the CVR Agreement to be executed at the Effective Time; |
• | the Rexahn Board’s consideration that the combined company will be led by an experienced senior management team from Ocuphire and a board of directors with representation from each of the current boards of directors of Rexahn and Ocuphire; |
• | the Rexahn Board’s consideration of the financial analysis of Oppenheimer and the opinion of Oppenheimer delivered to the Rexahn Board on June 17, 2020, to the effect that, as of the date of such opinion, and based upon and subject to the various assumptions made, procedures followed, matters considered and limitations and qualifications on the scope of the review undertaken by Oppenheimer, as set forth in its written opinion, the Exchange Ratio was fair to Rexahn Stockholders, from a financial point of view, and that Oppenheimer’s opinion was based on an estimated Exchange Ratio of 4.3820, which assumed Rexahn would deliver an estimated Parent Cash Amount of $720,000 on the Anticipated Closing Date, resulting in Rexahn Stockholders owning approximately 11.9% of the combined company immediately following consummation of the merger on a fully diluted basis; |
• | Rexahn’s recent results of operations and financial condition; and |
• | the terms of the Merger Agreement, the CVR Agreement, the Pre-Merger Financing transaction documents and associated transactions. |
• | the fact that the Exchange Ratio will be adjusted downward to the extent the Parent Cash Amount is below $3.2 million on the Anticipated Closing Date, and Rexahn’s belief, based on current estimates, that it is reasonably likely to deliver significantly less than $3.2 million on the Anticipated Closing Date; |
• | the fact that the Parent Cash Amount will be reduced by an estimated warrant liability amount to be calculated approximately ten days prior to the Closing, with such estimated warrant liabilities being impacted by, among other things, the stock price of Rexahn common stock on such calculation date; and |
• | the fact that all Rexahn Stockholders may be further diluted based on the price reset provisions and Investor Warrants contemplated by the Pre-Merger Financing and the recognition that the fairness opinion from Oppenheimer did not address the potential additional dilution as a result of such price reset provisions and Investor Warrants. |
• | historical and current information concerning Ocuphire’s business, including its financial performance and condition, operations, management and pre-clinical and clinical data; |
• | the potential value of Nyxol and APX3330 and the ability of the combined company to advance the development of the Nyxol and APX3330 programs; |
• | Ocuphire’s prospects if it were to remain an independent company, including its need to obtain additional financing to continue its operations and the terms on which it would be able to obtain such financing, if at all; |
• | the belief of the Ocuphire Board that no alternatives to the merger were reasonably likely to create greater value for stockholders after reviewing the various strategic options to enhance stockholder value that were considered by the Ocuphire Board; |
• | the potential to provide Ocuphire’s current stockholders with greater liquidity by owning stock in the combined company, a public company; |
• | the expectation that the merger with Rexahn would be a more time- and cost-efficient means to access capital than other options considered by and available to Ocuphire, including private placements, venture debt financings and traditional methods of accessing the public markets through an initial public offering of Ocuphire’s securities; |
• | the anticipated cash resources of the combined company expected to be available at the Closing and the anticipated burn rate of the combined company; |
• | the broader range of investors potentially available to the combined company as a public company to support the development of Ocuphire’s product candidates, as compared with the investors to which Ocuphire could otherwise gain access if it continued to operate as a privately held company; |
• | the ability to improve Ocuphire’s balance sheet through the conversion of the Ocuphire convertible notes and accrued interest into common stock; |
• | the expectation that substantially all of Ocuphire’s employees, particularly its management, will serve in similar roles at the combined company; |
• | the expectation that the merger will be treated as a tax-free reorganization for U.S. federal income tax purposes, with the result that Ocuphire Stockholders will not recognize taxable gain or loss for U.S. federal income tax purposes upon the exchange of Ocuphire common stock for Rexahn common stock pursuant to the merger; |
• | the terms and conditions of the Merger Agreement, including, without limitation, the following: |
• | the expected relative percentage ownership of Rexahn Stockholders and Ocuphire Stockholders in the combined company at the Closing and the implied valuation of Ocuphire and Rexahn; |
• | the parties’ representations, warranties and covenants and the conditions to their respective obligations; and |
• | the limited number and nature of the conditions of the obligation of Rexahn to consummate the merger; and |
• | the likelihood that the merger will be consummated on a timely basis. |
• | solicit, initiate or knowingly encourage, induce or facilitate the communication, making, submission or announcement of, any “acquisition proposal” or “acquisition inquiry” (each as defined below) or take any action that could reasonably be expected to lead to an acquisition proposal or acquisition inquiry; |
• | furnish any non-public information with respect to it to any person in connection with or in response to an acquisition proposal or acquisition inquiry; |
• | engage in discussions or negotiations with any person with respect to any acquisition proposal or acquisition inquiry; |
• | approve, endorse or recommend an acquisition proposal; |
• | execute or enter into any letter of intent or similar document or any contract contemplating or otherwise relating to any “acquisition transaction” as defined below (other than a confidentiality agreement permitted by the Merger Agreement); or |
• | publicly propose to do any of the above. |
Name | | | Age | | | Position |
Executive Officers | | | | | ||
Mina Sooch | | | 52 | | | President, Chief Executive Officer, Treasurer, Director, Vice Chair |
Bernhard Hoffmann | | | 65 | | | VP of Corporate Development and Finance, Secretary |
| | | | | |||
Non-Employee Directors | | | | | ||
Sean Ainsworth | | | 52 | | | Director, Lead Independent Director |
James S. Manuso | | | 72 | | | Director |
Cam Gallagher | | | 51 | | | Director, Chair of the Board |
Alan R. Meyer | | | 67 | | | Director |
Richard J. Rodgers | | | 53 | | | Director |
Susan K. Benton | | | 56 | | | Director |
• | Rexahn and Ocuphire securityholders may not realize a benefit from the merger commensurate with the ownership dilution they will experience in connection with the merger and the Pre-Merger Financing. |
• | The Exchange Ratio set forth in the Merger Agreement is adjustable based on the Parent Cash Amount, so the relative ownership of the combined company as between current Rexahn Stockholders and current Ocuphire Securityholders may change based on, among other things, Rexahn’s interim cash burn prior to the Closing and the estimated warrant liabilities associated with the Rexahn Warrants. |
• | The market price of Rexahn common stock will impact the estimated warrant liability amount in the calculation of the Parent Cash Amount, and if the market price of Rexahn common stock continues to increase, Rexahn may not be able to satisfy the minimum Parent Cash Amount requirement in the Merger Agreement or, if Ocuphire waives the minimum Parent Cash Amount condition, Rexahn Stockholders may own significantly less of the combined company than currently estimated. |
• | Rexahn has issued and may issue additional shares of Rexahn common stock or other Rexahn securities before Closing in exchange for outstanding Rexahn warrants, which has diluted and would further dilute the ownership interests of current Rexahn Stockholders. |
• | The merger consideration at the Closing may have a greater or lesser value than at the time the Merger Agreement was signed. |
• | Failure to complete the merger may result in either Rexahn or Ocuphire paying a termination fee to the other party and could significantly harm the market price of Rexahn common stock and negatively affect the future business and operations of each company. |
• | The issuance of Rexahn common stock to Ocuphire Stockholders pursuant to the Merger Agreement and the resulting change in control from the merger must be approved by Rexahn Stockholders, and the Merger Agreement and transactions contemplated thereby must be approved by the Ocuphire Stockholders. Failure to obtain these approvals would prevent the Closing. |
• | The merger may be completed even though certain events occur prior to the Closing that materially and adversely affect Rexahn or Ocuphire. |
• | Some Rexahn and Ocuphire officers and directors have interests in the merger that are different from the respective stockholders of Rexahn and Ocuphire and that may influence them to support or approve the merger without regard to the interests of the respective stockholders of Rexahn and Ocuphire. |
• | The market price of Rexahn common stock following the merger may decline as a result of the merger. |
• | Rexahn Stockholders and Ocuphire Securityholders will have a reduced ownership and voting interest in, and will exercise less influence over the management of, the combined company following the Closing as compared to their current ownership and voting interest in the respective companies. |
• | The combined company will need to raise additional capital by issuing securities or debt or through licensing or other strategic arrangements, which may cause dilution to the combined company's stockholders or restrict the combined company's operations or impact its proprietary rights. |
• | During the pendency of the merger, Rexahn and Ocuphire may not be able to enter into a business combination with another party at a favorable price because of restrictions in the Merger Agreement, which could adversely affect their respective businesses. |
• | Certain provisions of the Merger Agreement may discourage third parties from submitting alternative takeover proposals, including proposals that may be superior to the arrangements contemplated by the Merger Agreement. |
• | Because the lack of a public market for Ocuphire common stock makes it difficult to evaluate the value of Ocuphire common stock, the Ocuphire Stockholders may receive shares of Rexahn common stock in the merger that have a value that is less than, or greater than, the fair market value of Ocuphire common stock. |
• | If the conditions to the merger are not met or waived, the merger will not occur. |
• | Rexahn and Ocuphire do not anticipate that the combined company will pay any cash dividends in the foreseeable future. |
• | Future sales of shares by existing stockholders could cause the combined company’s stock price to decline. |
• | The Pre-Merger Financing may not be satisfied. |
• | Litigation relating to the merger could require Rexahn or Ocuphire to incur significant costs and suffer management distraction, and could delay or enjoin the merger. |
• | The historical unaudited pro forma condensed combined financial information may not be representative of the combined company’s results after the merger. |
• | Ocuphire’s risk-adjusted projections, which Oppenheimer relied on for its fairness opinion delivered to the Rexahn Board, assume that Ocuphire’s product candidates receive FDA approval. Ocuphire’s failure to obtain such FDA approval would adversely impact the combined company’s potential to generate revenue, its business and its results of operations. |
• | The opinion received by the Rexahn Board from Oppenheimer is subject to a number of assumptions and it has not been, and is not expected to be, updated to reflect changes in circumstances that may have occurred since the date of the opinion. |
| | | For the Year Ended December 31, | | | Six Months Ended June 30, | ||||||||||||||||
| | | 2019 | | | 2018 | | | 2017 | | | 2016 | | | 2015 | | | 2020 (unaudited) | | | 2019 (unaudited) | |
Statement of Operations Data: | | | | | | | | | | | | | | | |||||||
Revenues | | | $— | | | $— | | | $— | | | $— | | | $— | | | $1,150,000 | | | $— |
Operating expenses: | | | | | | | | | | | | | | | |||||||
General and administrative | | | 5,738,227 | | | 7,428,615 | | | 6,639,421 | | | 6,324,236 | | | 6,115,210 | | | 3,372,898 | | | 3,035,538 |
Research and development | | | 5,476,776 | | | 13,109,058 | | | 10,715,296 | | | 10,089,149 | | | 12,148,226 | | | 688,397 | | | 3,890,631 |
Total operating expenses | | | 11,215,003 | | | 20,537,673 | | | 17,354,717 | | | 16,413,385 | | | 18,263,436 | | | 4,061,295 | | | 6,926,169 |
Loss from operations | | | (11,215,003) | | | (20,537,673) | | | (17,354,717) | | | (16,413,385) | | | (18,263,436) | | | (2,911,295) | | | (6,926,169) |
Other income (expense), net | | | | | | | | | | | | | | | |||||||
Interest income | | | 313,700 | | | 254,344 | | | 207,003 | | | 118,565 | | | 103,269 | | | 40,461 | | | 178,035 |
Mediation settlement | | | — | | | — | | | — | | | 1,770,658 | | | — | | | — | | | — |
Unrealized gain (loss) on fair value of warrants | | | 2,265,869 | | | 5,546,049 | | | (7,594,162) | | | 5,529,907 | | | 3,986,727 | | | (227,094) | | | 1,940,854 |
Other, net | | | — | | | 368,750 | | | (552,627) | | | (313,090) | | | (211,116) | | | — | | | — |
Total other income (expense), net | | | 2,579,569 | | | 6,169,143 | | | (7,939,786) | | | 7,106,040 | | | 3,878,880 | | | (186,633) | | | 2,118,889 |
Net loss | | | $(8,635,434) | | | $(14,368,530) | | | $(25,294,503) | | | $(9,307,345) | | | $(14,384,556) | | | $(3,097,928) | | | $(4,807,280) |
Net Loss per share, basic and diluted(1) | | | $(2.18) | | | $(5.25) | | | $(11.10) | | | $(5.15) | | | $(9.49) | | | $(0.77) | | | $(1.23) |
Weighted average shares outstanding, basic and diluted (1) | | | 3,960,163 | | | 2,738,506 | | | 2,278,105 | | | 1,807,628 | | | 1,515,465 | | | 4,019,141 | | | 3,900,208 |
| | | As of December 31, | | | As of June 30, | ||||||||||||||||
| | | 2019 | | | 2018 | | | 2017 | | | 2016 | | | 2015 | | | 2020 (unaudited) | | | 2019 (unaudited) | |
Balance Sheet Data: | | | | | | | | | | | | | | | |||||||
Cash, cash equivalents and marketable securities | | | $12,216,767 | | | $14,725,821 | | | $26,831,095 | | | $20,315,580 | | | $23,439,526 | | | $9,208,951 | | | $16,260,169 |
Total assets | | | $12,968,772 | | | $16,042,926 | | | $28,287,881 | | | $21,043,532 | | | $24,805,029 | | | $10,249,074 | | | $17,677,234 |
Total liabilities | | | $3,010,818 | | | $5,480,036 | | | $11,519,285 | | | $3,985,070 | | | $6,029,481 | | | $3,245,761 | | | $4,054,128 |
Accumulated deficit | | | $(163,322,676) | | | $(154,687,242) | | | $(140,318,712) | | | $(115,024,209) | | | $(105,716,864) | | | $(166,420,604) | | | $(159,494,522) |
Total Stockholders’ Equity | | | $9,957,954 | | | $10,562,890 | | | $16,768,596 | | | $17,058,462 | | | $18,775,548 | | | $7,003,313 | | | $13,623,106 |
(1) | Basic loss per common share is computed by dividing net loss by the weighted average number of shares of common stock outstanding for the period. Diluted loss per common share is computed by dividing net loss by the weighted average number of shares of common stock outstanding, plus the number of common share equivalents that would be dilutive. On May 5, 2017, Rexahn effected a one-for-ten reverse stock split of the outstanding shares of Rexahn common stock, together with a corresponding proportional reduction in the number of authorized shares of Rexahn capital stock. Each 10 shares of Rexahn common stock, par value $0.0001 per share, issued and outstanding at the effective time of the reverse stock split were reclassified and combined into one share of common stock par value $0.0001 per share. On April 12, 2019, Rexahn effected a 1-for-12 reverse stock split of the outstanding shares of Rexahn common stock. Each 12 shares of Rexahn common stock, par value $0.0001 per share, issued and outstanding at the effective time of the reverse stock split were reclassified and combined into one share of common stock par value $0.0001 per share. All share and per share amounts have been restated for all periods to give retroactive effect to the reverse stock splits. |
| | | Year Ended December 31, | | | Six Months Ended June 30, | |||||||
Statement of Operations Data: | | | 2019 | | | 2018 | | | 2020 (unaudited) | | | 2019 (unaudited) |
Operating expenses: | | | | | | | | | ||||
General and administrative | | | $1,820,477 | | | $743,279 | | | $942,471 | | | $777,189 |
Research and development | | | 2,372,502 | | | 555,951 | | | 928,561 | | | 828,450 |
Acquired in-process research and development | | | — | | | — | | | 2,126,253 | | | — |
Total operating expenses | | | 4,192,979 | | | 1,299,230 | | | 3,997,285 | | | 1,605,639 |
Loss from operations | | | (4,192,979) | | | (1,299,230) | | | (3,997,285) | | | (1,605,639) |
Interest expense | | | (1,409,096) | | | (196,506) | | | (1,242,624) | | | (319,869) |
Fair value change of premium conversion derivatives | | | (499,414) | | | (21,238) | | | (721,444) | | | (132,083) |
Gain on note extinguishment | | | — | | | — | | | 1,260,350 | | | — |
Other income, net | | | (67,471) | | | (109,897) | | | 8,505 | | | — |
Loss before income taxes | | | (6,168,960) | | | (1,626,871) | | | (4,692,498) | | | (2,057,591) |
Benefit (provision) for income taxes | | | — | | | — | | | — | | | — |
Net loss | | | (6,168,960) | | | (1,626,871) | | | (4,692,498) | | | (2,057,591) |
Other comprehensive loss, net of tax | | | — | | | — | | | — | | | — |
Comprehensive loss | | | $(6,168,960) | | | $(1,626,871) | | | $(4,692,498) | | | $(2,057,591) |
Net loss per share: | | | | | | | | | ||||
Basic and diluted(1) | | | $(2.29) | | | $(0.68) | | | $(1.36) | | | $(0.77) |
Number of shares used in per share calculations: | | | | | | | | | ||||
Basic and diluted(1) | | | 2,692,793 | | | 2,388,941 | | | 3,451,031 | | | 2,685,467 |
(1) | See Note 9 to Ocuphire’s financial statements appearing elsewhere in this proxy statement/prospectus/information statement for further details on the calculation of net loss per share, basic and diluted, attributable to common stockholders, and the weighted-average number of shares used in computation of the per share amounts. |
| | | As of December 31, | | | As of June 30, | |||||||
| | | 2019 | | | 2018 | | | 2020 (unaudited) | | | 2019 (unaudited) | |
Balance Sheet Data: | | | | | | | | | ||||
Cash and cash equivalents | | | $1,536,917 | | | $451,342 | | | $854,331 | | | $1,191,794 |
Total assets | | | 1,784,279 | | | 533,073 | | | 3,499,908 | | | 2,996,731 |
Convertible notes (including premium conversion derivatives) | | | 8,380,498 | | | 1,643,146 | | | 10,300,593 | | | 5,848,940 |
Total liabilities | | | 9,343,803 | | | 2,231,747 | | | 12,277,261 | | | 6,596,510 |
Accumulated deficit | | | (8,054,703) | | | (1,885,743) | | | (12,747,201) | | | (3,943,334) |
Total shareholders’ deficit | | | (7,559,524) | | | (1,698,674) | | | (8,777,353) | | | (3,599,779) |
| | | For the Six Months Ended June 30, 2020 | | | For the Year Ended December 31, 2019 | |
Revenue | | | $1,150,000 | | | $— |
General and administrative expenses | | | 2,705,055 | | | 7,070,635 |
Research and development expenses | | | 1,616,958 | | | 7,849,278 |
Acquired in-process research and development expenses | | | 2,126,253 | | | — |
Loss from operations | | | (5,298,266) | | | (14,919,913) |
Net loss attributable to common stockholders | | | (5,476,394) | | | (12,407,815) |
Net loss per share, basic and diluted | | | (0.21) | | | (0.59) |
| | | As of June 30, 2020 | |
Cash and cash equivalents | | | $29,121,282 |
Working capital, net | | | 22,298,160 |
Total assets | | | 31,451,968 |
Accumulated deficit | | | (29,952,599) |
Total stockholders’ equity | | | 22,288,221 |
| | | Six Months Ended June 30, 2020 | | | Year Ended December 31, 2019 | |
Historical Per Common Share Data: | | | | | ||
Basic and diluted net loss per share | | | $(0.77) | | | $(2.18) |
Book value per share | | | 1.74 | | | 2.48 |
| | | Six Months Ended June 30, 2020 | | | Year Ended December 31, 2019 | |
Historical Per Common Share Data: | | | | | ||
Basic and diluted net loss per share | | | $(1.36) | | | $(2.29) |
Book value per share | | | (2.48) | | | (2.80) |
| | | Six Months Ended June 30, 2020 | | | Year Ended December 31, 2019 | |
Pro Forma Per Common Share Data: | | | | | ||
Basic and diluted net loss per share | | | $(0.21) | | | $(0.59) |
Book value per share | | | 0.85 | | | N/A |
• | general business, economic or political conditions affecting the industries in which Ocuphire or Rexahn, as applicable, operates; |
• | any natural disaster or any acts of war, armed hostilities or terrorism; |
• | changes in financial, banking or securities markets; |
• | with respect to Rexahn, any change in its stock price or trading volume excluding any underlying effect that may have caused such change, unless such effect is otherwise exempt from causing a material adverse effect under the Merger Agreement; |
• | any change in, or any compliance with or action taken for the purpose of complying with, applicable laws or U.S. GAAP, or interpretations thereof; |
• | continued losses from operations or decreases in cash balances of Rexahn; and |
• | the taking of any action, or failure to take action, by Rexahn or Ocuphire required to comply with the terms of the Merger Agreement. |
• | investors react negatively to the prospects of the combined company’s product candidates, business and financial condition following the merger; |
• | the effect of the merger on the combined company’s business and prospects is not consistent with the expectations of financial or industry analysts; or |
• | the combined company does not achieve the perceived benefits of the merger as rapidly or to the extent anticipated by financial or industry analysts. |
• | its ability to consummate the merger with Ocuphire; |
• | the level of development and commercialization efforts of BioSense and HaiChang and the receipt of milestone and other payments, if any, from such parties under their respective agreements with Rexahn; |
• | the scope, rate of progress and cost of its preclinical and clinical trials for any product candidate in its future pipeline and results of future clinical trials; |
• | the cost and timing of regulatory filings and approvals for any product candidates that successfully complete clinical trials; |
• | the timing and nature of any strategic transactions that Rexahn undertakes, including potential partnerships; |
• | the effect of competing technological and market developments; |
• | the cost incurred in responding to actions by activist stockholders; and |
• | the cost of filing, prosecuting, defending and enforcing its intellectual property rights. |
• | continued preclinical development and clinical trials for its product candidates; |
• | finding and maintaining suitable partnerships to help Rexahn research, develop and commercialize product candidates; |
• | efforts to seek regulatory approvals for its product candidates; |
• | implementing additional internal systems and infrastructure; and |
• | hiring additional personnel or entering into relationships with third parties to perform functions that Rexahn is unable to perform on its own. |
• | the attention of its remaining management and employees may be directed toward the completion of the merger and related matters and may be diverted from Rexahn’s day-to-day business operations; and |
• | third parties may seek to terminate or renegotiate their relationships with Rexahn as a result of the merger, whether pursuant to the terms of their existing agreements with Rexahn or otherwise. |
• | successfully conducting preclinical and clinical trials; |
• | obtaining regulatory approval; |
• | formulating and manufacturing products; and |
• | conducting sales and marketing activities. |
• | the cost of preclinical studies and clinical trials may be greater than Rexahn anticipates; |
• | delay or failure in reaching agreement with the FDA or a foreign regulatory authority on the design of a given trial, or in obtaining authorization to commence a trial, including due to a government shutdown, the COVID-19 pandemic, or future public health emergency; |
• | delay or failure in reaching agreement on acceptable terms with prospective contract research organizations (“CROs”) and clinical trial sites; |
• | delay or failure in obtaining approval of an institutional review board (“IRB”) to conduct a clinical trial at a given site; |
• | withdrawal of clinical trial sites from Rexahn’s clinical trials, including as a result of changing standards of care or the ineligibility of a site to participate, or due to COVID-19 pandemic; |
• | delay or failure in recruiting and enrolling study subjects, or the loss of study subjects, including due to the COVID-19 pandemic; |
• | delay or failure in having subjects complete a clinical trial or return for post-treatment follow up; |
• | clinical sites or investigators deviating from trial protocol, failing to conduct the trial in accordance with applicable regulatory requirements, or dropping out of a trial; |
• | inability to identify and maintain a sufficient number of trial sites; |
• | failure of third-party CROs to meet their contractual obligations or deadlines; |
• | the need to modify a study protocol; |
• | negative or inconclusive results during clinical trials, including the emergence of dosing issues, unforeseen safety issues or lack of effectiveness; |
• | changes in the standard of care of the indication being studied; |
• | reliance on third-party suppliers for the clinical trial supply of product candidates; |
• | inability to monitor patients adequately during or after treatment; |
• | lack of sufficient funding to finance the clinical trials; and |
• | changes in governmental regulations or administrative action. |
• | disagreement with the design or implementation of Rexahn’s clinical trials; |
• | failure to demonstrate to the authority’s satisfaction that the product candidate is safe and effective for the proposed indication; |
• | failure of clinical trial results to meet the level of statistical significance required for approval; |
• | failure to demonstrate that the product’s benefits outweigh its risks; |
• | disagreement with Rexahn’s interpretation of preclinical or clinical data; and |
• | inadequacies in the manufacturing facilities or processes of third-party manufacturers. |
• | Rexahn may suspend marketing of such product; |
• | regulatory authorities may withdraw their approvals of such product; |
• | regulatory authorities may require additional warnings on the label that could diminish the usage or otherwise limit the commercial success of such products; |
• | Rexahn may be required to develop a REMS for such product or, if a REMS is already in place, to incorporate additional requirements under the REMS, or to develop a similar strategy as required by a comparable foreign regulatory authority; |
• | Rexahn may be required to conduct post-market studies; |
• | Rexahn could be sued and held liable for harm caused to subjects or patients; and |
• | Rexahn’s reputation may suffer. |
• | awareness of a drug’s availability and benefits; |
• | perceptions by members of the health care community, including physicians, about the safety and effectiveness of Rexahn’s drugs; |
• | pharmacological benefit and cost-effectiveness of Rexahn’s products relative to competing products; |
• | availability of reimbursement for Rexahn’s products from government or other third-party payors; |
• | effectiveness of marketing and distribution efforts by Rexahn and Rexahn’s licensees and distributors, if any; and |
• | the price at which Rexahn sells its products. |
• | the federal Anti-Kickback Statute (the “Anti-Kickback Statute”) prohibits persons from, among other things, knowingly and willfully soliciting, offering, receiving or providing remuneration (interpreted to include anything of value), directly or indirectly, in cash or in kind, to induce or reward, or in return for, the referral of an individual for the furnishing or arranging for the furnishing, or the purchase, lease or order, or arranging for or recommending purchase, lease or order, any good or service for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid; |
• | the federal civil False Claims Act (the “FCA”) imposes penalties, including through civil whistleblower or qui tam actions, against individuals or entities for, among other things, knowingly presenting, or causing to be presented, claims for payment of government funds that are false or fraudulent or making a false record or statement material to an obligation to pay money to the government or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government; |
• | HIPAA imposes criminal liability for knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third party payers, knowingly and willfully embezzling or stealing from a health care benefit program, willfully obstructing a criminal investigation of a health care offense, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statements or representations or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry, in connection with the delivery of, or payment for, healthcare benefits, items or services. Similar to the Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation; |
• | HIPAA and its implementing regulations also impose obligations on certain covered entity health care providers, health plans and health care clearinghouses as well as their business associates that perform certain services involving the use or disclosure of individually identifiable health information, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
• | the federal Physician Payment Sunshine Act (the “Physician Payment Sunshine Act”), being implemented as the Open Payments Program, which requires manufacturers of drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to CMS information related to direct or indirect payments and other transfers of value to physicians and teaching hospitals (and certain other practitioners beginning in 2022), as well as ownership and investment interests held in the company by physicians and their immediate family members; and |
• | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; state and foreign laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to certain healthcare providers; state and foreign laws that require drug manufacturers to report information related to clinical trials, or information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws that govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
• | developing drugs; |
• | undertaking preclinical testing and human clinical trials; |
• | obtaining FDA and other regulatory approvals of drugs; |
• | formulating and manufacturing drugs; and |
• | launching, marketing and selling drugs. |
• | Rexahn may be unable to contract with third-party manufacturers on acceptable terms, or at all, because the number of potential manufacturers is limited. Potential manufacturers of any product candidate that is approved will be subject to FDA compliance inspections and any new manufacturer would have to be qualified to produce Rexahn’s products; |
• | Rexahn’s third-party manufacturers might be unable to formulate and manufacture Rexahn’s drugs in the volume and of the quality required to meet Rexahn’s clinical and commercial needs, if any; |
• | Rexahn’s third-party manufacturers may not perform as agreed or may not remain in the contract manufacturing business for the time required to supply Rexahn’s clinical trials through completion or to successfully produce, store and distribute Rexahn’s commercial products, if approved; |
• | Drug manufacturers are subject to ongoing periodic unannounced inspection by the FDA and other government agencies to ensure compliance with current good manufacturing practices (“cGMP”) and other government regulations and corresponding foreign standards. Rexahn does not have direct control over third-party manufacturers’ compliance with these regulations and standards, but Rexahn may ultimately be responsible for any of their failures; |
• | If any third-party manufacturer makes improvements in the manufacturing process for Rexahn’s products, Rexahn may not own, or may have to share, the intellectual property rights to such improvements; and |
• | A third-party manufacturer may gain knowledge from working with Rexahn that could be used to supply one of Rexahn’s competitors with a product that competes with Rexahn’s. |
• | the degree and range of protection any patents will afford Rexahn against competitors, including whether third parties find ways to invalidate or otherwise circumvent Rexahn’s licensed patents; |
• | if and when patents will issue in the United States or any other country; |
• | whether or not others will obtain patents claiming aspects similar to those covered by Rexahn’s licensed patents and patent applications; |
• | whether Rexahn will need to initiate litigation or administrative proceedings to protect Rexahn’s intellectual property rights, which may be costly whether Rexahn wins or loses; |
• | whether any of Rexahn’s patents will be challenged by Rexahn’s competitors alleging invalidity or unenforceability and, if opposed or litigated, the outcome of any administrative or court action as to patent validity, enforceability or scope; |
• | whether a competitor will develop a similar compound that is outside the scope of protection afforded by a patent or whether the patent scope is inherent in the claims modified due to interpretation of claim scope by a court; |
• | whether there were activities previously undertaken by a licensor that could limit the scope, validity or enforceability of licensed patents and intellectual property; or |
• | whether a competitor will assert infringement of its patents or intellectual property, whether or not meritorious, and what the outcome of any related litigation or challenge may be. |
• | obtain licenses, which may not be available on commercially reasonable terms, if at all; |
• | redesign its products or processes to avoid infringement; |
• | stop using the subject matter claimed in patents held by others, which could cause Rexahn to lose the use of one or more of Rexahn’s product candidates; |
• | pay damages; or |
• | defend litigation or administrative proceedings that may be costly whether Rexahn wins or loses and that could result in a substantial diversion of Rexahn’s management resources. |
• | Rexahn’s ability to consummate the transactions contemplated by the Merger Agreement, including the merger; |
• | the announcement of new products or product enhancements by Rexahn or its competitors; |
• | changes in Rexahn’s relationships with its licensors or other strategic partners; |
• | developments concerning intellectual property rights and regulatory approvals; |
• | variations in Rexahn’s and Rexahn’s competitors’ results of operations; |
• | changes in earnings estimates or recommendations by securities analysts; |
• | changes in the structure of healthcare payment systems; and |
• | developments and market conditions in the pharmaceutical and biotechnology industries, including due to the COVID-19 pandemic. |
• | the data collected from preclinical studies and clinical trials of Ocuphire’s product candidates may not be sufficient to support the submission of an NDA; |
• | Ocuphire may not be able to demonstrate to the satisfaction of the FDA that its product candidates are safe and effective for any indication; |
• | the results of clinical trials may not meet the level of statistical significance or clinical significance required by the FDA for approval; |
• | the FDA may disagree with the number, design, size, conduct, or implementation of Ocuphire’s clinical trials; |
• | the FDA may not find the data from preclinical studies and clinical trials sufficient to demonstrate that Ocuphire’s product candidates’ clinical and other benefits outweigh the safety risks; |
• | the FDA may disagree with Ocuphire’s interpretation of data from preclinical studies or clinical trials; |
• | the FDA may not accept data generated at Ocuphire’s clinical trial sites; |
• | the FDA may have difficulties scheduling an advisory committee meeting in a timely manner or the advisory committee may recommend against approval of Ocuphire’s application or may recommend that the FDA require, as a condition of approval, additional preclinical studies or clinical trials, limitations on approved labeling or distribution and use restrictions; |
• | the FDA may require development of a Risk Evaluation and Mitigation Strategy (REMS) as a condition of approval; |
• | the FDA may identify deficiencies in the manufacturing processes or facilities of third party manufacturers with which Ocuphire enters into agreements for clinical and commercial supplies; or |
• | the FDA may change its approval policies or adopt new regulations. |
• | regulators or IRBs may not authorize Ocuphire or its investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site including due to the ongoing COVID-19 pandemic or other public health emergency; |
• | government or regulatory delays and changes in regulatory requirements, policy and guidelines may require Ocuphire to perform additional clinical trials or use substantial additional resources to obtain regulatory approval, including due to the ongoing COVID-19 pandemic or other public health emergency; |
• | Ocuphire may have delays in reaching or fail to reach agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites, including due to the ongoing COVID-19 pandemic or other public health emergency; |
• | clinical trials may produce negative or inconclusive results, and Ocuphire may decide, or regulators may require it, to conduct additional clinical trials or abandon product development programs, including due to the ongoing COVID-19 pandemic or other public health emergency; |
• | the number of patients required for clinical trials may be larger, enrollment in these clinical trials may be slower or participants may drop out of these clinical trials at a higher rate than Ocuphire anticipates, including due to the ongoing COVID-19 pandemic or other public health emergency; |
• | Ocuphire’s third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to Ocuphire in a timely manner, or at all; |
• | Ocuphire’s patients or medical investigators may be unwilling to follow its clinical trial protocols; |
• | Ocuphire might have to suspend or terminate clinical trials for various reasons, including a finding that the participants are being exposed to unacceptable health risks; |
• | the cost of clinical trials may be greater than Ocuphire anticipates, including due to the ongoing COVID-19 pandemic or other public health emergency; |
• | the supply or quality of any product candidate or other materials necessary to conduct clinical trials may be insufficient or inadequate; |
• | the product candidate may have undesirable side effects or other unexpected characteristics, causing Ocuphire or its investigators, regulators or IRBs to suspend or terminate the trials; |
• | clinical trials may be delayed or terminated because of the ongoing COVID-19 pandemic or another public health emergency; and |
• | federal agencies may, due to reduced manpower or diverted resources to the COVID-19 pandemic, require more time to review clinical trial protocols and INDs. |
• | severity of the disease under investigation; |
• | availability and efficacy of medications already approved for the disease under investigation; |
• | eligibility criteria for the trial in question; |
• | competition for eligible patients with other companies conducting clinical trials for product candidates seeking to treat the same indication or patient population; |
• | its payments for conducting clinical trials; |
• | perceived risks and benefits of the product candidate under study; |
• | efforts to facilitate timely enrollment in clinical trials; |
• | patient referral practices of physicians; |
• | the ability to monitor patients adequately during and after treatment; |
• | proximity and availability of clinical trial sites for prospective patients; |
• | the ability of patients to safely participate in clinical trials during the COVID-19 pandemic or other public health emergencies; and |
• | the ability to monitor patients adequately during periods in which social distancing is required or recommended due to the COVID-19 pandemic. |
• | regulatory authorities may withdraw their approval of the product; |
• | Ocuphire may be required to recall the product, change the way this product is administered, conduct additional clinical trials, or change the labeling or distribution of the product (including REMS); |
• | additional restrictions may be imposed on the marketing of, or the manufacturing processes for, the product; |
• | Ocuphire may be subject to fines, injunctions, or the imposition of civil or criminal penalties; |
• | Ocuphire could be sued and held liable for harm caused to patients; |
• | the product may be rendered less competitive and sales may decrease; or |
• | Ocuphire’s reputation may suffer generally both among clinicians and patients. |
• | obtain favorable results from and complete the clinical development of both Nyxol and APX3330 for their planned indications, including successful completion of the Phase 2 and Phase 3 trials for these indications; |
• | submit an application to regulatory authorities for both product candidates and receive marketing approval in the United States and foreign countries; |
• | contract for the manufacture of commercial quantities of its product candidates at acceptable cost levels; |
• | establish sales and marketing capabilities to effectively market and sell its product candidates in the United States or other markets, alone or with a pharmaceutical partner; and |
• | achieve market acceptance of its product candidates in the medical community and with third-party payors. |
• | the scope, size, rate of progress, results, and costs of researching and developing its product candidates, and initiating and completing its preclinical studies and clinical trials; |
• | the cost, timing and outcome of its efforts to obtain marketing approval for its product candidates in the United States and other countries, including to fund the preparation and filing of an NDA with the FDA for its product candidates and to satisfy related FDA requirements and regulatory requirements in other countries; |
• | the number and characteristics of any additional product candidates it develops or acquires, if any; |
• | Ocuphire’s ability to establish and maintain collaborations on favorable terms, if at all; |
• | the amount of revenue, if any, from commercial sales, should its product candidates receive marketing approval; |
• | the costs associated with commercializing its product candidates, if Ocuphire receives marketing approval, including the cost and timing of developing sales and marketing capabilities or entering into strategic collaborations to market and sell its product candidates; |
• | the cost of manufacturing its product candidates or products Ocuphire successfully commercializes; and |
• | the costs associated with general corporate activities, such as the cost of filing, prosecuting and enforcing patent claims and making regulatory filings. |
• | litigation involving patients taking Ocuphire’s drugs; |
• | restrictions on such drugs, manufacturers, or manufacturing processes; |
• | restrictions on the labeling or marketing of a drug; |
• | restrictions on drug distribution or use; |
• | requirements to conduct post-marketing studies or clinical trials; |
• | warning letters or untitled letters; |
• | withdrawal of the drugs from the market; |
• | refusal to approve pending applications or supplements to approved applications that Ocuphire submits; |
• | product recall or public notification or medical product safety alerts to healthcare professionals; |
• | fines, restitution, or disgorgement of profits or revenues; |
• | suspension or withdrawal of marketing approvals; |
• | damage to relationships with any potential collaborators; |
• | unfavorable press coverage and damage to Ocuphire’s reputation; |
• | refusal to permit the import or export of drugs; |
• | product seizure; or |
• | injunctions or the imposition of civil or criminal penalties. |
• | the federal Anti-Kickback Statute prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid; |
• | the federal false claims and civil monetary penalties laws, including the civil False Claims Act, impose criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease, or conceal an obligation to pay money to the federal government; |
• | HIPAA imposes criminal and civil liability for, among other things, executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
• | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, and its implementing regulations, also imposes obligations, including mandatory contractual terms, on certain people and entities with respect to safeguarding the privacy, security, and transmission of individually identifiable health information; |
• | the federal Physician Payments Sunshine Act under the Affordable Care Act requires certain manufacturers of drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, with specific exceptions, to report specially to the Centers for Medicare & Medicaid Services within the U.S. Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests; and |
• | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, in addition to requiring drug manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures. Certain state and foreign laws also govern the privacy and security of health information in ways that differ from each other and often are not preempted by HIPAA, thus complicating compliance efforts. |
• | comply with the regulations of the FDA and applicable non-U.S. regulators; |
• | provide accurate information to the FDA and applicable non-U.S. regulators; |
• | comply with healthcare fraud and abuse laws and regulations in the United States and abroad; |
• | report financial information or data accurately; or |
• | disclose unauthorized activities to Ocuphire. |
• | Presbysol® (AGN-190584), with 1.25% pilocarpine, developed by Allergan plc. |
• | Presbidrops® (CSF-1), with low dose pilocarpine and a secondary agent (lubricant), developed by Orasis Pharmaceuticals Ltd. |
• | Liquid Vision®, with aceclidine (another miotic agent), developed by Presbyopia Therapies, LLC. |
• | MicroLine®, which is a microdose formulation of pilocarpine, developed by Eyenovia, Inc. |
• | KT-101, which uses pilocarpine in the AcuStream delivery system, developed by Kedalion Therapeutics, Inc. |
• | UNR844, which uses a mechanism that involves softening the lens to increase near visual acuity, developed by Novartis AG (originally Encore Vision, Inc.). |
• | Lucentis® (ranibizumab) and Avastin® (bevacizumab), which are anti-VEGF monoclonal antibody intravitreal injections, developed by Genentech, Inc. |
• | EYLEA® (aflibercept), a VEGF inhibitor intravitreal injection, developed by Regeneron Pharmaceuticals. |
• | Beovu® Brolucizumab, an anti-VEGF monoclonal antibody intravitreal injection, developed by Novartis AG. |
• | MACUGEN® (pegaptanib sodium injection), a selective inhibitor of VEGF-165, developed by Bausch + Lomb. |
• | Ozurdex® (dexamethasone), a corticosteroid IVT implant, developed by Allergan plc. |
• | Iluvien (fluocinolone acetonide), a corticosteroid IVT implant, developed by Alimera Sciences, Inc. |
• | There are also several pharmacological therapies in development, including: |
• | Abicipar pegol, an anti-VEGF intravitreal injection with a long duration of action, developed by Allergan plc and Molecular Partners. |
• | Farcimab, a bispecific antibody intravitreal injection that suppresses both VEGF and Angiopoietin-2, developed by Genentech, Inc. and Roche AG. |
• | KSI-301, an anti-VEGF antibody intravitreal injection coupled with a biopolymer that is intended to increase the time between injections, developed by Kodiak Sciences. |
• | OPT-302, an intravitreal injection which binds to multiple types of VEGF receptors that could be used with other anti-VEGF agents, developed by Opthea Limited. |
• | ALG-1001, an integrin peptide therapy intravitreal injection that is being evaluated as a sequential or in-combination therapy with bevacizumab in patients with DME, developed by Allegro Ophthalmics, LLC. |
• | the inability to recruit and retain adequate numbers of effective sales and marketing personnel or enter into distribution agreements with third parties; |
• | the inability of sales personnel to obtain access to physicians or persuade adequate numbers of physicians to prescribe its product candidate; |
• | the lack of complementary products to be offered by sales personnel, which may put Ocuphire at a competitive disadvantage relative to companies with more extensive product lines; |
• | unforeseen costs and expenses associated with creating an independent sales and marketing organization; and |
• | the inability to obtain sufficient coverage and reimbursement from third-party payors and governmental agencies. |
• | efficacy and potential advantages compared to alternative treatments; |
• | the ability to offer Ocuphire’s product for sale at competitive prices; |
• | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
• | any restrictions on the use of Ocuphire’s product together with other medications; |
• | interactions of its product with other medicines patients are taking; |
• | inability of certain types of patients to take Ocuphire’s product; |
• | demonstrated ability to treat patients and, if required by any applicable regulatory authority in connection with the approval for target indications as compared with other available therapies; |
• | the relative convenience and ease of administration as compared with other treatments available for approved indications; |
• | the prevalence and severity of any adverse side effects; |
• | limitations or warnings contained in the labeling approved by the FDA; |
• | availability of alternative treatments already approved or expected to be commercially launched in the near future; |
• | the effectiveness of Ocuphire’s sales and marketing strategies; |
• | Ocuphire’s ability to increase awareness through marketing efforts; |
• | guidelines and recommendations of organizations involved in research, treatment and prevention of various diseases that may advocate for alternative therapies; |
• | Ocuphire’s ability to obtain sufficient third-party coverage and adequate reimbursement; |
• | the willingness of patients to pay out-of-pocket in the absence of third-party coverage; and |
• | physicians or patients may be reluctant to switch from existing therapies even if potentially more effective, safe or convenient. |
• | decreased demand for any product candidate that Ocuphire is developing; |
• | injury to Ocuphire’s reputation and significant negative media attention; |
• | withdrawal of clinical trial participants; |
• | increased FDA warnings on product labels; |
• | significant costs to defend the related litigation; |
• | substantial monetary awards to trial participants or patients; |
• | distraction of management’s attention from Ocuphire’s primary business; |
• | loss of revenue; and |
• | the inability to commercialize any product candidate that Ocuphire may develop. |
• | have staffing difficulties; |
• | fail to comply with contractual obligations; |
• | experience regulatory compliance issues; |
• | undergo changes in priorities or become financially distressed; or |
• | form relationships with other entities, some of which may be Ocuphire’s competitors. |
• | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
• | collaborators may not perform their obligations as expected; |
• | collaborators may not pursue development and commercialization or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in the collaborator’s strategic focus or available funding, or external factors such as an acquisition that diverts resources or creates competing priorities; |
• | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials, or require a new formulation of a product candidate for clinical testing; |
• | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with its product candidate if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more attractive than Ocuphire’s; |
• | a collaborator with marketing and distribution rights to one or more product candidates may not commit sufficient resources to the marketing or distribution of any such product candidate; |
• | collaborators may not properly maintain or defend Ocuphire’s intellectual property rights or may use its proprietary information in such a way as to invite litigation that could jeopardize or invalidate Ocuphire’s proprietary information or expose Ocuphire to litigation; |
• | collaborators may infringe the intellectual property rights of third parties, which may expose Ocuphire to litigation and potential liability; |
• | disputes may arise between the Ocuphire and collaborators that result in the delay or termination of research, development, or commercialization of its product candidates, or in litigation or arbitration that diverts management attention and resources; |
• | Ocuphire may lose certain valuable rights under circumstances identified in its collaborations, including if it undergoes a change of control; |
• | collaborations may be terminated and such terminations may create a need for additional capital to pursue further development or commercialization of the applicable product candidates; |
• | collaborators may learn about Ocuphire’s discoveries and use this knowledge to compete with Ocuphire in the future; |
• | the results of collaborators’ preclinical or clinical studies could harm or impair other development programs; |
• | there may be conflicts between different collaborators that could negatively affect those collaborations and potentially others; |
• | the number and nature of Ocuphire’s collaborations could adversely affect its attractiveness to potential future collaborators or acquirers; |
• | collaboration agreements may not lead to development or commercialization of its product candidate in the most efficient manner or at all. If a present or future collaborator of Ocuphire were to be involved in a business combination, the continued pursuit and emphasis on its product development or commercialization program under such collaboration could be delayed, diminished, or terminated; and |
• | collaborators may be unable to obtain the necessary marketing approvals. |
• | increased operating expenses and cash requirements; |
• | the assumption of indebtedness or contingent liabilities; |
• | the issuance of Ocuphire’s equity securities which would result in dilution to Ocuphire Stockholders; |
• | assimilation of operations, intellectual property, products and product candidates of an acquired company, including difficulties associated with integrating new personnel; |
• | the diversion of management’s attention from Ocuphire’s existing product candidates and initiatives in pursuing such an acquisition or strategic partnership; |
• | retention of key employees, the loss of key personnel, and uncertainties in Ocuphire’s ability to maintain key business relationships; |
• | risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing products or product candidates and regulatory approvals; and |
• | Ocuphire’s inability to generate revenue from acquired intellectual property, technology and/or products sufficient to meet its objectives or even to offset the associated transaction and maintenance costs. |
• | any of Ocuphire’s patents, or any of its pending patent applications, if issued, will include claims having a scope sufficient to protect its product candidates; |
• | any of its pending patent applications will result in issued patents; |
• | Ocuphire will be able to successfully commercialize its product candidates, if approved, before its relevant patents expire; |
• | Ocuphire was the first to make the inventions covered by each of its patents and pending patent applications; |
• | Ocuphire was the first to file patent applications for these inventions; |
• | others will not develop similar or alternative technologies that do not infringe Ocuphire’s patents; |
• | any of Ocuphire’s patents will be valid and enforceable; |
• | any patents issued to Ocuphire will provide a basis for an exclusive market for its commercially viable products, will provide Ocuphire with any competitive advantages or will not be challenged by third parties; |
• | Ocuphire will develop additional proprietary technologies or product candidates that are separately patentable; or |
• | that Ocuphire’s commercial activities or products will not infringe upon the patents of others. |
• | the scope of rights granted under the license agreement and other interpretation-related issues; |
• | the extent to which Ocuphire’s product candidates, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
• | the sublicensing of patent and other rights under Ocuphire’s collaborative development relationships; |
• | Ocuphire’s diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
• | the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property; and |
• | the priority of invention of patented technology. |
• | compliance with differing or unexpected regulatory requirements for its product candidates; |
• | different medical practices and customs affecting acceptance of its product candidates, if approved, or any other approved product in the marketplace; |
• | language barriers; |
• | the interpretation of contractual provisions governed by foreign law in the event of a contract dispute; |
• | difficulties in staffing and managing foreign operations, and an inability to control commercial or other activities where it is relying on third parties; |
• | workforce uncertainty in countries where labor unrest is more common than in the United States; |
• | potential liability under the Foreign Corrupt Practice Act of 1977 or comparable foreign regulations; |
• | production shortages resulting from any events affecting raw material supply or manufacturing capability abroad; |
• | foreign government taxes, regulations, and permit requirements; |
• | U.S. and foreign government tariffs, trade restrictions, price and exchange controls, and other regulatory requirements; |
• | economic weakness, including inflation, natural disasters, war, events of terrorism, or political instability in particular foreign countries; |
• | fluctuations in currency exchange rates, which could result in increased operating expenses and reduced revenues; |
• | compliance with tax, employment, immigration, and labor laws, regulations, and restrictions for employees living or traveling abroad; |
• | changes in diplomatic and trade relationships; and |
• | challenges in enforcing its contractual and intellectual property rights, especially in those foreign countries that do not respect and protect intellectual property rights to the same extent as the United States. |
• | interruption in global manufacturing and shipping that has affected, and may continue to affect the transport of clinical trial materials and materials, including testing equipment and personal protective equipment; |
• | changes in local regulations as part of a response to the COVID-19 coronavirus outbreak which may result in unexpected costs; |
• | delay in the timing of interactions with the FDA due to absenteeism by federal employees or by the diversion of their efforts and attention to approval of other therapeutics or other activities related to COVID-19; |
• | impacts on Ocuphire’s ability to secure additional financing on favorable terms; and |
• | modifications to the Ocuphire convertible notes. |
• | the expected benefits of, and potential value created by, the merger for the securityholders of Rexahn and Ocuphire; |
• | the likelihood of the satisfaction of certain conditions to the completion of the merger, including the Pre-Merger Financing condition, and whether and when the merger will be consummated and the listing of the Rexahn common stock to be issued on the Nasdaq Capital Market; |
• | the expected amount of the Rexahn Parent Cash Amount on the Anticipated Closing Date and Rexahn’s ability to control and correctly estimate its operating expenses, the estimated warrant liability and its expenses associated with the merger, including litigation expenses; |
• | any statements regarding the effects of the Investor Warrants and the adjustment mechanisms in the Pre-Merger Financing on the ownership percentages of the Ocuphire Stockholders and Rexahn Stockholders in the combined company; |
• | the expected number of Rexahn securities included in the fully diluted number of Rexahn’s outstanding shares for purposes of calculating the Exchange Ratio; |
• | the impact of the coronavirus outbreak on the business of Rexahn and Ocuphire; |
• | any statements of the plans, strategies and objectives of management for future operations, including the execution of integration plans and the anticipated timing of filings; |
• | any statements of plans to develop and commercialize additional products candidates including planned preclinical, clinical, regulatory, and manufacturing activities; |
• | any statements concerning the attraction and retention of highly qualified personnel; |
• | any statements concerning the ability to protect and enhance the combined company’s products and intellectual property; |
• | any statements concerning developments and projections relating to the combined company’s competitors or industry; |
• | any statements concerning the combined company’s financial performance; |
• | any statements regarding expectations concerning Rexahn’s or Ocuphire’s relationships and actions with third parties; and |
• | future regulatory, judicial and legislative changes in Rexahn’s or Ocuphire’s industry. |
• | If Rexahn’s stock price continues to increase, the estimated liabilities associated with the Rexahn Warrants will continue to increase and Rexahn may not be able to meet the minimum Parent Cash Amount on the Anticipated Closing Date or the Rexahn Stockholders may own significantly less of the combined company than currently estimated; |
• | Rexahn and Ocuphire stockholders may not realize a benefit from the merger commensurate with the ownership dilution they will experience in connection with the merger and the Pre-Merger Financing; |
• | Rexahn’s issuance of additional shares of Rexahn common stock or other securities prior to Closing; |
• | the merger consideration may have greater or lesser value at the Closing than at the time the Merger Agreement is signed; |
• | failure to complete the merger may result in either party paying a termination fee or expenses to the other party and could harm the future business and operations of each company; |
• | if the conditions to the merger are not met, including failure to timely or at all obtain stockholder approval for the merger, failure to consummate the Pre-Merger Financing, or failure to comply with the continued listing standards of Nasdaq, the merger may not occur; |
• | the timing of the consummation of the merger is uncertain as is the ability of each of Rexahn and Ocuphire to consummate the merger; |
• | the merger may be completed even though material adverse changes may occur; |
• | Rexahn may not be able to correctly estimate its operating expenses and its expenses associated with the merger and may have a significantly lower Parent Cash Amount on the Anticipated Closing Date than currently estimated; |
• | Rexahn may not be able to maintain its Nasdaq listing until the Closing; |
• | as a result of any adjustments in the Exchange Ratio and the Pre-Merger Financing, Rexahn Stockholders or Ocuphire Stockholders may own less of the combined company than is currently anticipated; |
• | executive officers and directors of each company have interests in the merger that are different from yours, which may cause them to support or approve the merger without regard to your interests; |
• | the market price of Rexahn common stock may decline following the merger; |
• | conditions to payment under the CVRs may not be met and the CVRs may never deliver any value to the Rexahn Stockholders; |
• | restrictions in the Merger Agreement may prevent Rexahn and Ocuphire from entering into a business combination with another party at a favorable price; |
• | certain provisions of the Merger Agreement may discourage third parties from submitting alternative takeover proposals, including proposals that may be superior to the arrangements contemplated by the Merger Agreement; |
• | the Ocuphire Stockholders may receive consideration in the merger that is greater or less than the fair market value of the Ocuphire shares due to the lack of a public market for Ocuphire shares; |
• | if the merger does not qualify as a tax-free reorganization for U.S. federal income tax purposes, the receipt of Rexahn common stock pursuant to the merger could be fully taxable to all Ocuphire Stockholders; |
• | the combined company may never earn a profit; |
• | the combined company will be subject to the uncertainties associated with the clinical development and regulatory approval of its product candidates including potential delays in the commencement, enrollment and completion of clinical trials and that the results of prior clinical trials may not be predictive of future results; |
• | the combined company will be required to raise additional funds to finance its operations and remain a going concern and may be required to do so sooner than it expects; |
• | the combined company may not be able to raise additional funds when necessary, and/or on acceptable terms; |
• | the combined company’s small public float, low market capitalization, limited operating history, and lack of revenue may make it difficult and expensive for the combined company to raise additional funds; |
• | the pro forma combined financial statements may not be an indication of the combined company’s financial condition or results of operations following the completion of the merger and the transactions contemplated thereby; |
• | Rexahn and Ocuphire may not be able to protect their respective intellectual property rights; |
• | there may be changes in expected or existing competition for the combined company’s product candidates; |
• | the merger will result in changes to the combined company’s board of directors that may affect the combined company’s business strategy and operations; |
• | both companies expect the price of the combined company’s common stock may be volatile and may fluctuate substantially following the merger and the transactions contemplated thereby; |
• | if the combined company were to be delisted from Nasdaq, it could reduce the visibility, liquidity and price of its common stock; |
• | a significant portion of the combined company’s total outstanding shares of common stock may be sold into the public market at any point, which could cause the market price of the combined company’s common stock to drop significantly, even if the combined company is doing well; |
• | there may be adverse reactions or changes in business relationships resulting from announcement or completion of the merger; |
• | the combined company will have broad discretion in the use of its cash reserves and may not use them effectively; |
• | the combined company expects to continue to incur increased costs as a result of operating as a public company, and its management will be required to devote substantial time to compliance initiatives and corporate governance practices; |
• | the combined company does not anticipate paying any cash dividends on its capital stock in the foreseeable future; |
• | provisions in the combined company’s certificate of incorporation, its bylaws or Delaware law might discourage, delay or prevent a change in control of the company or changes in its management, which may depress the price of its common stock; |
• | the coronavirus (COVID-19) pandemic may have an adverse effect on the business of Rexahn, Ocuphire and the combined company, the medical community and the global economy; and |
• | securities analysts’ published reports could cause a decline in the price of the combined company’s stock. |
1. | to consider and vote upon a proposal to approve the issuance of Rexahn common stock to Ocuphire Stockholders pursuant to the Merger Agreement, a copy of which is attached as Annex A to this proxy statement/prospectus/information statement, and the change of control of Rexahn resulting from the merger under Nasdaq rules; |
2. | to consider and vote upon an amendment to the Rexahn Certificate of Incorporation to effect the Rexahn Reverse Stock Split at a ratio within the range of 1-for-3 to 1-for-5, with such specific ratio to be approved by the Rexahn Board, in the form attached as Annex B to this proxy statement/prospectus/information statement; |
3. | to consider and vote upon an amendment to the Rexahn Certificate of Incorporation to effect the Rexahn Name Change, in the form attached as Annex C to this proxy statement/prospectus/information statement; |
4. | to consider and vote upon a proposal to approve the adoption of the Ocuphire 2020 Plan, in the form attached as Annex D to this proxy statement/prospectus/information statement; |
5. | to consider and vote upon a proposal to approve the issuance of: (a) shares of Rexahn common stock upon the exercise of the Investor Warrants to be issued in the Pre-Merger Financing, and (b) additional shares of Rexahn common stock that may be issued following the closing of the Pre-Merger Financing, in each case pursuant to the Securities Purchase Agreement and as required by and in accordance with Nasdaq Listing Rule 5635; |
6. | to consider and vote upon an adjournment of the Rexahn special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of Proposal Nos. 1, 2, 3, 4 or 5; and |
7. | to transact such other business as may properly come before the Rexahn special meeting or any adjournment or postponement thereof. |
• | The Rexahn Board has determined that the issuance of Rexahn common stock to Rexahn Stockholders pursuant to the Merger Agreement and the change of control of Rexahn resulting from the merger are fair to, advisable and in the best interest of Rexahn and Rexahn Stockholders and has approved such items. The Rexahn Board recommends that Rexahn Stockholders vote “FOR” Proposal No. 1 to approve the issuance of Rexahn common stock to Ocuphire Stockholders and the change of control of Rexahn resulting from the merger. |
• | The Rexahn Board has determined that the Rexahn Reverse Stock Split is fair to, advisable and in the best interest of Rexahn and Rexahn Stockholders and has approved the Rexahn Reverse Stock Split. The Rexahn Board recommends that Rexahn Stockholders vote “FOR” Proposal No. 2 to approve an amendment to the Rexahn Certificate of Incorporation effecting the Rexahn Reverse Stock Split. |
• | The Rexahn Board has determined that the Rexahn Name Change is fair to, advisable and in the best interest of Rexahn and Rexahn Stockholders and has approved the Rexahn Name Change. The Rexahn Board recommends that Rexahn Stockholders vote “FOR” Proposal No. 3 to approve an amendment to the Rexahn Certificate of Incorporation effecting the Rexahn Name Change. |
• | The Rexahn Board has determined that the adoption of the Ocuphire 2020 Plan is fair to, advisable and in the best interest of Rexahn and Rexahn Stockholders and has approved the Ocuphire 2020 Plan. The Rexahn Board recommends that Rexahn Stockholders vote “FOR” Proposal No. 4 to approve the Ocuphire 2020 Plan. |
• | The Rexahn Board has determined that it is fair to, advisable, and in the best interests of Rexahn and Rexahn Stockholders and has approved the issuance of: (a) shares of Rexahn common stock upon the exercise of the Investor Warrants to be issued in the Pre-Merger Financing, and (b) additional shares of Rexahn common stock that may be issued following the closing of the Pre-Merger Financing, in each case pursuant to the Securities Purchase Agreement and as required by and in accordance with Nasdaq Listing Rule 5635. The Rexahn Board recommends that Rexahn Stockholders vote “FOR” Proposal No. 5 to approve the issuance of additional shares of Rexahn common stock in connection with the Pre-Merger Financing. |
• | The Rexahn Board has determined and believes that adjourning the Rexahn special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of Proposal Nos. 1, 2, 3, 4 or 5 is advisable and in the best interests of Rexahn and Rexahn Stockholders and has approved and adopted the proposal. The Rexahn Board recommends that Rexahn Stockholders vote “FOR” Proposal No. 6 to adjourn the Rexahn special meeting, if necessary, to solicit additional proxies if there are not sufficient votes in favor of Proposal Nos. 1, 2, 3, 4 or 5. |
• | to vote in person, attend the Rexahn special meeting and Rexahn will provide you a ballot when you arrive. |
• | to vote using the proxy card, simply mark, sign and date your proxy card and return it promptly in the postage-paid envelope provided. If you return your signed proxy card to Rexahn before the Rexahn special meeting, Rexahn will vote your shares as you direct on the proxy card. |
• | to vote by telephone or on the Internet, dial the number on the proxy card or visit the website on the proxy card form to complete an electronic proxy card. You will be asked to provide Rexahn’s number and control number from the enclosed proxy card. Your vote must be received by 11:59 p.m., Eastern Time on Friday, October 30, 2020 to be counted. |
• | Hogan Lovells provided materials regarding the fiduciary duties of directors in connection with the consideration of the merger transaction; |
• | the Rexahn Board received a report from Hogan Lovells regarding the legal due diligence review of Ocuphire and key provisions of the transaction documents, including the provisions regarding calculation of the Exchange Ratio, treatment of Rexahn’s and Ocuphire’s convertible securities in the merger, non-solicitation clause and fiduciary duty exceptions, termination provisions and related fee and expense reimbursement requirements, CVR terms, lock-up and voting agreements, and the terms of and requirements related to Pre-Merger Financing; and |
• | the Rexahn Board engaged in discussions relating to Ocuphire and the terms of the proposed merger and Pre-Merger Financing. |
• | the Rexahn Board received a presentation from Oppenheimer regarding its financial analyses of the Exchange Ratio, and stated its oral opinion, to be confirmed in writing in the same form as the draft opinion that had been previously provided to the Rexahn Board, that based upon the various assumptions, qualifications and limitations set forth therein, the Exchange Ratio is fair, from a financial point of view, to the Rexahn Stockholders; |
• | the Rexahn Board engaged in discussions relating to Ocuphire and the terms of the proposed merger, related transactions and Pre-Merger Financing; |
• | the Rexahn Board discussed and considered the factors and other items referenced in the section entitled “The Merger—Rexahn Reasons for the Merger”, including (i) Rexahn’s recent results of operations and financial condition, (ii) Rexahn’s business, operational and financial prospects in light of its clinical trial results to date, (iii) the costs of continuing to operate Rexahn’s business, including the costs associated with conducting future clinical development programs and acquiring new product candidates, (iv) the need to raise significant additional capital for future clinical and commercial development of Rexahn’s current and future product candidates, and the difficulty Rexahn has had raising capital in light of Rexahn’s low market capitalization and capitalization structure, and (v) the expectation that, given Rexahn’s existing cash resources, there would be a limited amount of available cash left, if any, to be distributed to Rexahn Stockholders in a potential dissolution or liquidation of Rexahn and the risks, costs of timing of such process; and |
• | after further discussion, the Rexahn Board unanimously determined that it was advisable and fair to, and in the best interests of Rexahn and the Rexahn Stockholders to enter into the Merger Agreement, approved the Merger Agreement and declared it advisable. |
• | the Rexahn Board’s belief that a go-it-alone scenario poses significant risk, including the risk of dilution to the Rexahn Stockholders, taking into account Rexahn’s business, operational and financial prospects, including its cash position, the limited value given by the marketplace to Rexahn’s product |
• | the Rexahn Board’s belief, given the risks associated with clinical development, and based in part on the judgment, advice and analysis of Rexahn senior management with respect to the potential strategic, financial and operational benefits of the merger (which judgment was informed in part by the business, technical, financial and legal due diligence investigation performed by Rexahn with respect to Ocuphire) that Ocuphire’s Phase 3 ready, lead product candidate, Nyxol, for multiple front-of-the-eye (pupil/cornea) indications, as well as its product candidate, APX3330, for multiple back-of-the-eye (retina) conditions, along with the experience of its management and other personnel, and the granting of CVRs to Rexahn Stockholders to provide a potential financial benefit in the event that any of Rexahn’s existing intellectual property is sold or licensed during a future period or Rexahn receives any payments from BioSense or HaiChang, would create more value for Rexahn Stockholders in the long term than Rexahn could create as an independent stand-alone company; |
• | the Rexahn Board’s review of the current development plans of Ocuphire to confirm the likelihood that the combined company would possess sufficient resources, or have access to sufficient resources, to allow Ocuphire senior management to focus on its plans for the continued development of Ocuphire’s product pipeline; |
• | the Rexahn Board’s consideration that the combined company should have sufficient cash at the Closing for the combined company to sustain its operations for the next eighteen months at the time of its consideration and the combined company’s public company structure will provide it with access to the public market to raise additional funds in the future; |
• | the Rexahn Board’s consideration of the results of its strategic review process, which included Oppenheimer’s outreach to 50 companies and the receipt of five inbound inquiries, resulting in the receipt of indications of interest from 19 companies. Further, the Rexahn Board’s consideration of the valuation and business prospects of all other strategic transaction candidates involved in its strategic review process, and its collective view that Ocuphire was the most attractive candidate for Rexahn due to, among other things, Ocuphire’s Phase 3 ready asset, Nyxol, was well as its APX3330 product candidate, Ocuphire’s strong financial position that includes backing from a syndicate of investors, the strength of Ocuphire’s management team, the potential market opportunity for Nyxol and APX3330, Ocuphire’s understanding of the potential value of Rexahn’s partnerships with BioSense and HaiChang, and that Ocuphire’s potential to achieve key milestones over the next several years could enable the combined company to access the public markets for additional financial resources; |
• | the Rexahn Board’s conclusion that the merger provides existing Rexahn Stockholders a significant opportunity to participate in the potential growth of the combined company following the merger, while potentially receiving certain cash payments from the grant, sale or transfer of rights to Rexahn’s existing intellectual property or pursuant to payments received by BioSense or HaiChang during a certain period following Closing on account of the CVR Agreement to be executed at the Effective Time; |
• | the Rexahn Board’s consideration that the combined company will be led by an experienced senior management team from Ocuphire and a board of directors with representation from each of the current boards of directors of Rexahn and Ocuphire; and |
• | the Rexahn Board’s consideration of the financial analysis of Oppenheimer and the opinion of Oppenheimer delivered to the Rexahn Board on June 17, 2020, to the effect that, as of the date of such opinion, and based upon and subject to the various assumptions made, procedures followed, matters considered and limitations and qualifications on the scope of the review undertaken by Oppenheimer, as set forth in its written opinion, the Exchange Ratio was fair to Rexahn Stockholders, from a financial point of view, and that Oppenheimer’s opinion was based on an estimated Exchange Ratio of 4.3820, which assumed Rexahn would deliver an estimated Parent Cash Amount of $720,000 on the Anticipated Closing Date, resulting in Rexahn Stockholders owning approximately 11.9% of the combined company immediately following consummation of the merger on a fully diluted basis. |
• | the limited value given by the marketplace to Rexahn’s product portfolio as evidenced by, among other things, the depressed trading price of Rexahn common stock, Rexahn’s low market capitalization, the market’s reaction to Rexahn’s recent clinical development announcements and Rexahn’s recent efforts to raise additional capital on reasonable terms; |
• | the lack of sufficient capital to complete additional preclinical studies and clinical trials on new indications or to acquire new product candidates, as well as the challenge of raising sufficient capital to complete studies and trials under terms that would be more favorable to Rexahn Stockholders than the merger with Ocuphire; |
• | the loss of operational capabilities of Rexahn and risks associated with continuing to operate Rexahn on a stand-alone basis, including Rexahn’s current limited number of employees and reliance on outside consultants and third-party contractors for ongoing clinical development activities; |
• | despite significant business development efforts, the inability of Rexahn to identify a pharmaceutical partner willing to provide significant financial support to co-develop or buy Rexahn’s assets; |
• | despite significant business development efforts, the inability of Rexahn to identify a pharmaceutical product or candidate to acquire; |
• | the market prices, volatility and trading volume of Rexahn common stock and current financial market conditions; |
• | the limited amount of available cash expected to be left, if any, to be distributed to Rexahn Stockholders in a potential dissolution and liquidation of Rexahn and the risks, costs and timing of such a process; and |
• | Rexahn’s potential inability to maintain its listing on the Nasdaq Capital Market without completing the merger. |
• | the fact that the Exchange Ratio, which is expected to result in Rexahn Stockholders immediately prior to the merger owning approximately 11.2% of the combined company immediately following the merger, on a fully-diluted basis, assuming Rexahn delivers at least $0 net cash on the Anticipated Closing Date, subject to adjustment based on Rexahn’s net cash on the Anticipated Closing Date, is financially attractive in light of Rexahn’s stand-alone value, recent stock price and strategic alternatives, and the potential value of Ocuphire following the merger; |
• | the rights of, and limitations on, Rexahn and Ocuphire under the Merger Agreement to consider certain unsolicited acquisition proposals under the certain circumstances, should such party receive a “superior offer”; |
• | the Rexahn Board’s belief that the terms of the Merger Agreement, including the parties’ representations, warranties and covenants, deal protection provisions and the conditions are reasonable for a transaction of this nature; and |
• | the Rexahn Board’s belief that the CVR Agreement potentially providing certain cash payments from the grant, sale or transfer of rights to Rexahn’s pre-closing intellectual property assets and from payments received by BioSense and HaiChang during a certain period following Closing to Rexahn Stockholders of record as of the closing, is reasonable and fair under the circumstances. |
• | the fact that the Exchange Ratio will be adjusted downward to the extent Rexahn’s Parent Cash Amount on the Anticipated Closing Date is below $3.2 million, and Rexahn’s belief, based on current estimates, that it is reasonably likely to deliver significantly less than $3.2 million on the Anticipated Closing Date; |
• | the fact that Rexahn’s Parent Cash Amount on the Anticipated Closing Date will be reduced by an estimated warrant liability amount to be calculated ten days prior to the Closing, with such estimated warrant liabilities being impacted by, among other things, the stock price and volatility of the Rexahn common stock. |
• | the fact that all Rexahn Stockholders may be further diluted based on the price reset provisions and Investor Warrants contemplated by the Pre-Merger Financing and the recognition that the fairness opinion from Oppenheimer did not address the potential additional dilution as a result of such price reset provisions and Investor Warrants; |
• | the $750,000 termination fee payable by Rexahn to Ocuphire upon the occurrence of certain events and the potential effect of such termination fee in deterring other potential acquirers from proposing an alternative transaction that may be more advantageous to Rexahn Stockholders; |
• | the $750,000 termination fee payable by Ocuphire to Rexahn upon the occurrence of certain events and the likelihood the receipt of the termination fee from Ocuphire will only offset a portion of the expenses incurred by Rexahn in connection with the merger; |
• | the substantial expenses incurred and to be incurred by Rexahn in connection with the merger; |
• | the possible volatility of the trading price of the Rexahn common stock resulting from the announcement of the merger and the effect such volatility could have on the causing additional dilution to all Rexahn Stockholders following the merger pursuant to the price reset provisions and Investor Warrants contemplated by the Pre-Merger Financing; |
• | the risks that the merger might not be consummated in a timely manner or at all and the potential effect of the public announcement of the merger or failure to complete the merger on the reputation of Rexahn; |
• | the risks to Rexahn’s business, operations and financial results in the event that the merger is not consummated; |
• | the strategic direction of the combined company following the Closing, which will be determined by a combination of individuals from Ocuphire senior management and the Ocuphire Board, including their ability to determine to discontinue any efforts to develop, sell or license any of Rexahn’s existing assets; and |
• | the various other risks associated with the combined company and the merger, including those described in the sections entitled “Risk Factors” and “Cautionary Statement Concerning Forward-Looking Statements”. |
• | Historical and current information concerning Ocuphire’s business, including its financial performance and condition, operations, management and pre-clinical and clinical data; |
• | The potential value of Nyxol and APX3330 and the ability of the combined company to advance the development of the Nyxol and APX3330 programs; |
• | Ocuphire’s prospects if it were to remain an independent company, including its need to obtain additional financing to continue its operations and the terms on which it would be able to obtain such financing, if at all; |
• | The belief of the Ocuphire Board that no alternatives to the merger were reasonably likely to create greater value for stockholders after reviewing the various strategic options to enhance stockholder value that were considered by the Ocuphire Board; |
• | The potential to provide Ocuphire’s current stockholders with greater liquidity by owning stock in the combined company, a public company; |
• | The expectation that the merger with Rexahn would be a more time- and cost-efficient means to access capital than other options considered by and available to Ocuphire, including private placements, venture debt financings and traditional methods of accessing the public markets through an initial public offering of Ocuphire’s securities; |
• | The anticipated cash resources of the combined company expected to be available at the Closing and the anticipated burn rate of the combined company; |
• | The broader range of investors potentially available to the combined company as a public company to support the development of Ocuphire’s product candidates, as compared with the investors to which Ocuphire could otherwise gain access if it continued to operate as a privately held company; |
• | The ability to improve Ocuphire’s balance sheet through the conversion of the Ocuphire convertible notes and accrued interest into common stock; |
• | The expectation that substantially all of Ocuphire’s employees, particularly its management, will serve in similar roles at the combined company; |
• | The expectation that the merger will be treated as a tax-free reorganization for U.S. federal income tax purposes, with the result that Ocuphire Stockholders will not recognize taxable gain or loss for U.S. federal income tax purposes upon the exchange of Ocuphire common stock for Rexahn common stock pursuant to the merger; |
• | The terms and conditions of the Merger Agreement, including, without limitation, the following: |
• | The expected relative percentage ownership of Rexahn Stockholders and Ocuphire Stockholders in the combined company at the Closing and the implied valuation of Ocuphire and Rexahn; |
• | The parties’ representations, warranties and covenants and the conditions to their respective obligations; and |
• | The limited number and nature of the conditions of the obligation of Rexahn to consummate the merger; and |
• | The likelihood that the merger will be consummated on a timely basis. |
• | The risk that the potential benefits of the Merger Agreement may not be realized; |
• | The risk that future sales of common stock by existing Rexahn Stockholders may cause the price of Rexahn common stock to fall, thus reducing the potential value of Rexahn common stock received by Ocuphire Stockholders following the merger; |
• | The termination fee of $750,000 and/or expense reimbursement of up to $750,000 payable by Ocuphire to Rexahn upon the occurrence of certain events, and the potential effect of such termination fee and expense reimbursement in deterring other potential acquirers from proposing a competing transaction that may be more advantageous to Ocuphire Stockholders; |
• | The price volatility of Rexahn common stock, which may reduce the potential value of Rexahn common stock received by Ocuphire Stockholders following the merger; |
• | The potential reduction of Rexahn’s net cash prior to Closing; |
• | The possibility that Rexahn could, under certain circumstances, consider unsolicited acquisition proposals if superior to the merger or change its recommendation to approve the merger upon certain events; |
• | The possibility that the merger might not be completed for a variety of reasons, such as the failure of Rexahn to obtain the required stockholder vote, and the potential adverse effect on the reputation of Ocuphire and the ability of Ocuphire to obtain financing in the future in the event the merger is not completed; |
• | The risk that the merger might not be consummated in a timely manner or at all; |
• | The expenses to be incurred in connection with the merger and related administrative challenges associated with combining the companies; |
• | The additional expenses that Ocuphire’s business will be subject to as a public company following the Closing to which it has not previously been subject; and |
• | Various other risks associated with the combined company and the merger, including the risks described in the section entitled “Risk Factors” beginning on page 35 of this proxy statement/prospectus/information statement. |
• | reviewed the financial terms of the merger described in the draft Merger Agreement, dated June 17, 2020 (the “Draft Merger Agreement”) and a draft of the Contingent Value Rights Agreement, dated June 17, 2020 (the “Draft CVR Agreement”), that would be executed in connection with the consummation of the merger. Both the Draft Merger Agreement and the Draft CVR Agreement were the most recent drafts made available to Oppenheimer prior to the delivery of its opinion; |
• | reviewed certain information, including certain projected financial information, relating to the business, earnings, and prospects of Rexahn and Ocuphire that was furnished to Oppenheimer by Rexahn and Ocuphire; |
• | conducted discussions with members of senior management and representatives of Rexahn and Ocuphire concerning the matters described in the second bullet above; |
• | reviewed the pro forma ownership structure of the combined entity resulting from the merger; |
• | reviewed publicly available information relating to the businesses of Rexahn and Ocuphire; |
• | reviewed and analyzed certain publicly available information concerning the terms (including financial terms) of selected merger and acquisition transactions and other business combinations that Oppenheimer considered relevant to its analysis; |
• | reviewed and analyzed certain publicly available information relating to selected companies that Oppenheimer deemed relevant to its analysis; |
• | performed a discounted cash flow analysis of the future cash flows of Ocuphire based upon financial projections for Ocuphire prepared by management of Ocuphire and approved for Oppenheimer’s use for such purpose by management of Rexahn (the “Ocuphire Projections”); |
• | reviewed the latest Pre-Merger Financing term sheets resulting from a marketed process of the contemplated Pre-Merger Financing conducted by Canaccord and Cantor (the “Pre-Merger Financing Term Sheet”); |
• | reviewed such other information, performed such other analyses, financial studies and investigations, and considered such other factors as Oppenheimer deemed appropriate for the purpose of rendering its opinion; and |
• | took into account Oppenheimer’s assessment of general economic, market and financial conditions and Oppenheimer’s experience in other transactions, as well as its experience in securities valuations and its knowledge of Rexahn’s and Ocuphire’s industries. |
Company Name | | | Total Enterprise Value |
Oyster Point Pharma, Inc. | | | $682.9 |
Aldeyra Therapeutics, Inc. | | | $119.8 |
Outlook Therapeutics, Inc. | | | $115.9 |
KalVista Pharmaceuticals, Inc. | | | $103.0 |
Aerpio Pharmaceuticals, Inc.* | | | $19.1 |
* | This current value reflects a recent COVID-19 partnership and, therefore, was excluded from high, mean, median and low calculations set forth below. |
| | | Total Enterprise Value | |
High | | | $682.9 |
Mean | | | $255.4 |
Median | | | $117.8 |
Low | | | $103.0 |
| | | Total Equity Value | |
Reference Low | | | $127.3 |
Reference High | | | $150.9 |
Announced Date | | | Target | | | Acquiror | | | Total Enterprise Value |
01/29/19 | | | Helio Vision | | | Aldeyra Therapeutics | | | $25.0 |
05/08/17 | | | River Vision Development | | | Horizon Therapeutics | | | $470.0 |
12/20/16 | | | Encore Vision | | | Novartis | | | $456.0 |
10/26/16 | | | Ocular Technologies Sarl | | | Sun Ophthalmics Inc. | | | $40.0* |
08/11/16 | | | ForSight VISION5 | | | Allergan | | | $220.0 |
08/03/15 | | | Foresight Biotherapeutics | | | Shire | | | $300.0 |
* | Ocular Technologies’ transaction value only depicts upfront payments as the milestones were not disclosed and, therefore, were excluded from the mean and median calculations set forth below. |
| | | Total Enterprise Value | |
Mean | | | $294.2 |
Median | | | $300.0 |
| | | Total Equity Value | |
Reference Low | | | $291.3 |
Reference High | | | $351.3 |
Total Enterprise Value | |||||||||
| | | Perpetuity Growth Method | |||||||
Discount Rate | | | (2.5)% | | | (2.0)% | | | (1.5%) |
12.0% | | | $244.4 | | | $245.7 | | | $247.0 |
13.0% | | | $211.6 | | | $212.5 | | | $213.5 |
14.0% | | | $183.3 | | | $184.0 | | | $184.7 |
| | | Total Equity Value | |
Reference Low | | | $204.5 |
Reference High | | | $268.2 |
| | | Total Equity Value | |
Reference Low | | | $108.0 |
Reference High | | | $132.0 |
Low Rexahn Share Price | | | $1.31 |
High Rexahn Share Price | | | $3.66 |
Low Rexahn Market Capitalization | | | $5.3 |
High Rexahn Market Capitalization | | | $14.7 |
Low Ocuphire Implied Equity Value | | | $127.3 |
High Ocuphire Implied Equity Value | | | $150.9 |
Low Implied Exchange Ratio | | | 5.1367 |
High Implied Exchange Ratio | | | 17.0081 |
Low Implied Rexahn Pro Forma Equity Ownership | | | 3.4% |
High Implied Rexahn Pro Forma Equity Ownership | | | 10.4% |
Low Rexahn Share Price | | | $1.31 |
High Rexahn Share Price | | | $3.66 |
Low Rexahn Market Capitalization | | | $5.3 |
High Rexahn Market Capitalization | | | $14.7 |
Low Ocuphire Implied Equity Value | | | $291.3 |
High Ocuphire Implied Equity Value | | | $351.3 |
Low Implied Exchange Ratio | | | 11.7528 |
High Implied Exchange Ratio | | | 39.6006 |
Low Implied Rexahn Pro Forma Equity Ownership | | | 1.5% |
High Implied Rexahn Pro Forma Equity Ownership | | | 4.8% |
Low Rexahn Share Price | | | $1.31 |
High Rexahn Share Price | | | $3.66 |
Low Rexahn Market Capitalization | | | $5.3 |
High Rexahn Market Capitalization | | | $14.7 |
Low Ocuphire Implied Equity Value | | | $204.5 |
High Ocuphire Implied Equity Value | | | $268.2 |
Low Implied Exchange Ratio | | | 8.2534 |
High Implied Exchange Ratio | | | 30.2411 |
Low Implied Rexahn Pro Forma Equity Ownership | | | 1.9% |
High Implied Rexahn Pro Forma Equity Ownership | | | 6.7% |
Low Rexahn Share Price | | | $1.31 |
High Rexahn Share Price | | | $3.66 |
Low Rexahn Market Capitalization | | | $5.3 |
High Rexahn Market Capitalization | | | $14.7 |
Low Ocuphire Implied Equity Value | | | $108.0 |
High Ocuphire Implied Equity Value | | | $132.0 |
Low Implied Exchange Ratio | | | 4.3581 |
High Implied Exchange Ratio | | | 14.8818 |
Low Implied Rexahn Pro Forma Equity Ownership | | | 3.8% |
High Implied Rexahn Pro Forma Equity Ownership | | | 12.0% |
• | Only the U.S. market was considered. |
• | Projections were made for Nxyol in NVD, RM and presbyopia and for APX3330 in DR/DME. The amounts projected for revenues and cost items were risk-adjusted, as described below. |
• | The assumed price of Nyxol was $600 annually per patient for NVD, $500 annually per patient for presbyopia, and $5 per use for RM. |
• | The assumed price of APX3330 was $5,000 annually per patient. |
• | Nyxol was assumed to receive FDA approval in 2023 for NVD and RM and 2024 for presbyopia, and APX3330 was assumed to receive FDA approval in 2024, with commercial launch beginning one year later for each. |
• | Launch costs were assumed to be $50.0 million for each indication, before probability risk adjustments. |
• | Following commercialization, cost of goods sold, SG&A expenditures and R&D expenditures as a percentage of revenue were assumed to be 10.0%, 35.0% and 10.0%, respectively. |
• | The cumulative risk-adjusted probabilities of success provided by Ocuphire management were 30%, 57%, 16% and 11% for NVD, RM, presbyopia and DR/DME, respectively. |
| | | 2020 | | | 2021 | | | 2022 | | | 2023 | | | 2024 | | | 2025 | | | 2026 | | | 2027 | | | 2028 | | | 2029 | | | 2030 | |
Inflow | | | | | | | | | | | | | | | | | | | | | | | |||||||||||
Total Sales | | | — | | | — | | | — | | | — | | | $27.9 | | | $67.2 | | | $110.2 | | | $182.2 | | | $268.3 | | | $318.2 | | | $338.6 |
Outflow | | | | | | | | | | | | | | | | | | | | | | | |||||||||||
COGS | | | — | | | — | | | — | | | — | | | $2.8 | | | $6.7 | | | $11.0 | | | $18.2 | | | $26.8 | | | $31.8 | | | $33.9 |
SG&A(1) | | | $8.1 | | | $8.1 | | | $8.1 | | | $8.1 | | | $9.8 | | | $23.5 | | | $38.6 | | | $63.7 | | | $93.9 | | | $111.4 | | | $118.5 |
Clinical Cost (risk adjusted)(2) | | | $12.3 | | | $13.6 | | | $6.0 | | | $0.3 | | | $40.7 | | | $14.5 | | | $0.7 | | | $1.7 | | | $2.6 | | | $4.5 | | | $4.2 |
Other R&D Expense(3) | | | $3.0 | | | $— | | | $3.0 | | | $6.0 | | | $5.8 | | | $6.7 | | | $11.0 | | | $18.2 | | | $26.8 | | | $31.8 | | | $33.9 |
EBIT | | | ($23.4) | | | ($21.7) | | | ($17.1) | | | ($14.4) | | | ($31.2) | | | $15.7 | | | $48.9 | | | $80.3 | | | $118.2 | | | $138.6 | | | $148.2 |
Cash Taxes(4) | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | $8.4 | | | $30.4 | | | $35.6 | | | $38.1 |
Net Free Cash Flow | | | ($23.4) | | | ($21.7) | | | ($17.1) | | | ($14.4) | | | ($31.2) | | | $15.7 | | | $48.9 | | | $72.0 | | | $87.8 | | | $103.0 | | | $110.1 |
Net Change in Working Capital | | | — | | | — | | | — | | | — | | | $4.4 | | | $6.2 | | | $6.8 | | | $11.5 | | | $13.6 | | | $7.9 | | | $3.2 |
Adjusted Net Free Cash Flow | | | ($23.4) | | | ($21.7) | | | ($17.1) | | | ($14.4) | | | ($35.6) | | | $9.5 | | | $42.1 | | | $60.5 | | | $74.1 | | | $95.1 | | | $106.9 |
| | | 2031 | | | 2032 | | | 2033 | | | 2034 | | | 2035 | | | 2036 | | | 2037 | | | 2038 | | | 2039 | | | 2040 | |
Inflow | | | | | | | | | | | | | | | | | | | | | ||||||||||
Total Sales | | | $341.9 | | | $345.4 | | | $348.8 | | | $352.3 | | | $122.3 | | | $123.6 | | | $124.8 | | | $126.0 | | | $127.3 | | | $128.6 |
Outflow | | | | | | | | | | | | | | | | | | | | | ||||||||||
COGS | | | $34.3 | | | $34.5 | | | $34.9 | | | $35.2 | | | $12.2 | | | $12.4 | | | $12.6 | | | $12.6 | | | $12.7 | | | $12.9 |
SG&A(1) | | | $119.7 | | | $120.9 | | | $122.1 | | | $123.3 | | | $42.8 | | | $43.2 | | | $43.7 | | | $44.1 | | | $44.6 | | | $45.0 |
Clinical Cost (risk adjusted)(2) | | | $4.2 | | | $4.3 | | | $4.3 | | | $4.3 | | | $0.4 | | | $— | | | $— | | | $— | | | $— | | | $— |
Other R&D Expense(3) | | | $34.2 | | | $34.5 | | | $34.9 | | | $35.2 | | | $12.2 | | | $12.3 | | | $12.5 | | | $12.6 | | | $12.7 | | | $12.9 |
EBIT | | | $149.6 | | | $151.2 | | | $152.7 | | | $154.2 | | | $54.6 | | | $55.5 | | | $56.2 | | | $56.7 | | | $57.3 | | | $57.9 |
Cash Taxes | | | $38.6 | | | $38.8 | | | $39.2 | | | $39.6 | | | $14.0 | | | $14.3 | | | $14.4 | | | $14.6 | | | $14.7 | | | $14.9 |
Net Free Cash Flow | | | $111.2 | | | $112.3 | | | $113.4 | | | $114.6 | | | $40.6 | | | $41.3 | | | $41.7 | | | $42.1 | | | $42.6 | | | $43.0 |
Net Change in Working Capital | | | $0.5 | | | $0.5 | | | $0.5 | | | $0.7 | | | ($36.5) | | | $0.2 | | | $0.2 | | | $0.2 | | | $0.2 | | | $0.2 |
Adjusted Net Free Cash Flow | | | $110.6 | | | $111.8 | | | $112.9 | | | $114.0 | | | $77.1 | | | $41.1 | | | $41.5 | | | $41.9 | | | $42.4 | | | $42.8 |
(1) | Includes overhead costs of $8.1 million annually in 2020 through 2023. |
(2) | Includes pre-commercialization risk-adjusted clinical costs, launch costs and post-commercialization royalty payments. |
(3) | Includes (i) preclinical costs and manufacturing development costs of $1.0 million and $2.0 million in 2020, respectively, (ii) NDA regulatory fees and other costs of $3.0 million, $6.0 million and $3.0 million in 2022, 2023, and 2024, respectively, and (iii) assumed R&D expenditures at 10% of revenue post-commercialization. |
(4) | Net operating loss carryforwards are assumed in 2020 through 2026, deferring the payment of cash taxes until 2027. |
| | | Number of Shares Underlying Unexercised Options Exercisable | | | Number of Shares Underlying Unexercised Options Unexercisable | |
Executive Officers | | | | | ||
Douglas J. Swirsky | | | 32,984 | | | 29,515 |
Non-Employee Directors | | | | | ||
Peter Brandt | | | 11,887 | | | — |
Charles Beever | | | 11,721 | | | — |
Kwang Soo Cheong | | | 11,721 | | | — |
Richard J. Rodgers | | | 10,639 | | | — |
Ben Gil Price | | | 6,672 | | | 6,271 |
Lara Sullivan | | | 5,897 | | | 4,720 |
• | a reduction of his salary or target bonus percentage; |
• | a relocation requiring him to be based at any office that is more than 35 miles from Rexahn’s office at the time of the signing of the agreement; |
• | any material breach by Rexahn of the terms and provisions of the agreement or any other material agreement between Mr. Swirsky and Rexahn; or |
• | a material diminution in his duties or authority inconsistent with his position. |
• | the Effective Time occurred on September 10, 2020; |
• | a price per share of Rexahn common stock of $3.01, which represents the average closing trading price of Rexahn common stock over the first five business days following the first public announcement of the merger; |
• | the employment of the named executive officer will be terminated on such date in a manner that entitles the named executive officer to receive the severance payments and benefits under the terms of the employment agreement with the named executive officer (as described above). The employment of the named executive officer is expected to be terminated effective immediately after the Closing; and |
• | the named executive officer does not enter into a new agreement or otherwise becomes legally entitled to, prior to the Effective Time, additional compensation or benefits. |
Name | | | Cash ($)(1) | | | Equity ($)(2) | | | Prerequisites/ Benefits ($)(3) | | | Total ($) |
Douglas J. Swirsky | | | $1,168,750 | | | — | | | $11,705 | | | $1,180,455 |
Ely Benaim(4) | | | — | | | — | | | — | | | — |
Lisa Nolan(4) | | | — | | | — | | | — | | | — |
(1) | The cash payment to Mr. Swirsky is set forth in his employment agreement, as described above and is payable in a lump sum if Rexahn consummates the merger and Rexahn terminates Mr. Swirsky’s employment without Cause in accordance with the terms of the Merger Agreement or Mr. Swirsky terminates for Good Reason provided that Mr. Swirsky has executed and delivered to Rexahn a general release. |
(2) | None of Mr. Swirsky’s option awards were in-the-money as of September 10, 2020. |
(3) | The amounts in this column represent (i) 401(k) employer matching contributions to be paid to Mr. Swirsky and (ii) 18 months of COBRA premiums Rexahn intends to provide to Mr. Swirsky. |
(4) | Dr. Benaim resigned from Rexahn in March 2019, and Dr. Nolan resigned from Rexahn in September 2019 |
| | | Amount Invested in Pre-Merger Financing | | | Number of Initial Shares Issuable | |
Executive Officers | | | | | ||
Mina Sooch | | | $65,000 | | | 3,609 |
Non-Employee Directors | | | | | ||
Sean Ainsworth | | | $50,000 | | | 2,776 |
Alan R. Meyer | | | $10,000 | | | 555 |
James S. Manuso | | | $25,000 | | | 1,388 |
Cam Gallagher | | | $50,000 | | | 2,776 |
Richard Rodgers | | | $100,000 | | | 5,553 |
| | | Note Value of Ocuphire Convertible Notes | | | Number of Shares of Ocuphire common stock Underlying Ocuphire Convertible Notes | |
Executive Officers | | | | | ||
Mina Sooch | | | $398,262 | | | 22,429 |
Bernhard Hoffmann | | | $43,155 | | | 2,430 |
Non-Employee Directors | | | | | ||
Sean Ainsworth | | | $194,370 | | | 10,946 |
Alan R. Meyer | | | $453,689 | | | 28,250 |
James S. Manuso | | | $143,620 | | | 8,088 |
Cam Gallagher | | | $191,340 | | | 10,775 |
| | | Number of Shares of Common Stock Underlying Options As of September 10, 2020 | | | Number of Vested Shares of Common Stock Underlying Options As of September 10, 2020 | |
Executive Officers | | | | | ||
Mina Sooch | | | 318,750 | | | 230,750 |
Bernhard Hoffmann | | | 73,075 | | | 53,875 |
Non-Employee Directors | | | | | ||
Sean Ainsworth | | | 83,756 | | | 48,756 |
Alan R. Meyer | | | 63,756 | | | 46,156 |
James S. Manuso | | | 83,756 | | | 45,956 |
Cam Gallagher | | | 83,756 | | | 45,956 |
• | each share of Ocuphire common stock outstanding immediately prior to the Effective Time (excluding certain shares to be canceled pursuant to the Merger Agreement and shares held by holders of Ocuphire common stock who have exercised and perfected appraisal rights or dissenters’ rights as more fully described in the section entitled “The Merger—Appraisal Rights and Dissenters’ Rights” in this proxy statement/prospectus/information statement) will automatically be converted into the right to receive a number of shares of Rexahn common stock equal to the Exchange Ratio, subject to adjustment to account for the Rexahn Reverse Stock Split and as described below (prior to the Effective Time, the outstanding Ocuphire convertible notes will be converted into Ocuphire common stock and will participate in the merger on the same basis as the other shares of Ocuphire common stock); and |
• | each option to purchase shares of Ocuphire common stock outstanding and unexercised immediately prior to the Effective Time will be assumed by Rexahn and will become an option, subject to vesting, to purchase shares of Rexahn common stock with the number of shares of Rexahn common stock underlying such options and the exercise prices for such options adjusted to reflect the Exchange Ratio and the Rexahn Reverse Stock Split. |
• | “Aggregate Valuation” means the sum of (i) the Ocuphire Valuation, plus (ii) the Rexahn Valuation. |
• | “Ocuphire Allocation Percentage” means the quotient (expressed as a percentage, with the percentage rounded to two decimal places) determined by dividing (i) the Ocuphire Valuation by (ii) the Aggregate Valuation. |
• | “Ocuphire Merger Shares” means the product determined by multiplying (i) the Post-Closing Rexahn Shares by (ii) the Ocuphire Allocation Percentage. |
• | “Ocuphire Outstanding Shares” means the total number of shares of Ocuphire capital stock outstanding immediately prior to the Effective Time expressed on a fully-diluted and as-converted to Ocuphire common stock basis, and assuming, without limitation or duplication, (i) the exercise of all Ocuphire Options outstanding as of immediately prior to the Effective Time, (ii) the conversion of all Ocuphire convertible notes and other outstanding indebtedness, (iii) the Closing of the Pre-Merger Financing and (iv) the issuance of shares of Ocuphire capital stock in respect of all other outstanding options, |
• | “Ocuphire Valuation” means $120,000,000. |
• | “Rexahn Allocation Percentage” means the quotient (expressed as a percentage, with the percentage rounded to two decimal places) determined by dividing (i) the Rexahn Valuation by (ii) the Aggregate Valuation. |
• | “Rexahn Outstanding Shares” means the total number of shares of Rexahn common stock outstanding immediately prior to the Effective Time expressed on a fully-diluted basis and as converted to Rexahn common stock basis, with any in-the-money Replacement Warrants calculated based on the treasury stock method, and (i) assuming, without limitation or duplication, the exercise of all Replacement Warrants (subject to sub-clause (ii)(e) below and the settlement in shares of each in-the-money Rexahn Option outstanding as of the Effective Time solely to the extent such Rexahn Option will not be canceled at or prior to the Effective Time or exercised prior thereto, and (ii) without regard to and excluding (a) any Rexahn Options canceled at or prior to the Effective Time, (b) any out-of-the-money Rexahn Options granted under the Rexahn 2003 Plan, (c) any Rexahn Warrants that have been or will be exercised, exchanged, cancelled and/or terminated before closing, (d) any out-of-the-money Rexahn Warrants (e) one-half of each share of Rexahn common stock underlying any out-of-the money Replacement Warrants, and (f) any shares of Rexahn common stock reserved for future issuance pursuant to Rexahn stock plans. A Rexahn Option or Rexahn Warrant is out-of-the-money if its exercise price is equivalent to or greater than $2.5025, and is in-the-money if its exercise price is less than such amount. |
• | “Rexahn Valuation” means $20,000,000 (the “Rexahn Base Valuation”); provided, however, to the extent that (i) the Parent Cash Amount is less than $3,200,000, then the Rexahn Base Valuation shall be reduced by $150,000 for each $100,000 that the Parent Cash Amount as so determined is less than $3,200,000, subject to a minimum Rexahn valuation of $12,000,000; and (ii) the Parent Cash Amount is greater than $6,000,000, then the Rexahn Base Valuation shall be increased by $150,000 for each $100,000 that the Parent Cash Amount as so determined is greater than $6,000,000. |
• | “Post-Closing Rexahn Shares” means the quotient determined by dividing (i) the Rexahn Outstanding Shares by (ii) the Rexahn Allocation Percentage. |
Parent Cash Amount ($) | | | Rexahn Valuation ($) | | | Rexahn Allocation Percentage | | | Ocuphire Allocation Percentage |
3,200,000 | | | 20,000,000 | | | 14.29% | | | 85.71% |
3,100,000 | | | 19,850,000 | | | 14.19% | | | 85.81% |
3,000,000 | | | 19,700,000 | | | 14.10% | | | 85.90% |
2,900,000 | | | 19,550,000 | | | 14.01% | | | 85.99% |
2,800,000 | | | 19,400,000 | | | 13.92% | | | 86.08% |
2,700,000 | | | 19,250,000 | | | 13.82% | | | 86.18% |
2,600,000 | | | 19,100,000 | | | 13.73% | | | 86.27% |
2,500,000 | | | 18,950,000 | | | 13.64% | | | 86.36% |
2,400,000 | | | 18,800,000 | | | 13.54% | | | 86.46% |
2,300,000 | | | 18,650,000 | | | 13.45% | | | 86.55% |
2,200,000 | | | 18,500,000 | | | 13.36% | | | 86.64% |
2,100,000 | | | 18,350,000 | | | 13.26% | | | 86.74% |
Parent Cash Amount ($) | | | Rexahn Valuation ($) | | | Rexahn Allocation Percentage | | | Ocuphire Allocation Percentage |
2,000,000 | | | 18,200,000 | | | 13.17% | | | 86.83% |
1,900,000 | | | 18,050,000 | | | 13.07% | | | 86.93% |
1,800,000 | | | 17,900,000 | | | 12.98% | | | 87.02% |
1,700,000 | | | 17,750,000 | | | 12.89% | | | 87.11% |
1,600,000 | | | 17,600,000 | | | 12.79% | | | 87.21% |
1,500,000 | | | 17,450,000 | | | 12.70% | | | 87.30% |
1,400,000 | | | 17,300,000 | | | 12.60% | | | 87.40% |
1,300,000 | | | 17,150,000 | | | 12.50% | | | 87.50% |
1,200,000 | | | 17,000,000 | | | 12.41% | | | 87.59% |
1,100,000 | | | 16,850,000 | | | 12.31% | | | 87.69% |
1,000,000 | | | 16,700,000 | | | 12.22% | | | 87.78% |
900,000 | | | 16,550,000 | | | 12.12% | | | 87.88% |
800,000 | | | 16,400,000 | | | 12.02% | | | 87.98% |
700,000 | | | 16,250,000 | | | 11.93% | | | 88.07% |
600,000 | | | 16,100,000 | | | 11.83% | | | 88.17% |
500,000 | | | 15,950,000 | | | 11.73% | | | 88.27% |
400,000 | | | 15,800,000 | | | 11.63% | | | 88.37% |
300,000 | | | 15,650,000 | | | 11.54% | | | 88.46% |
200,000 | | | 15,500,000 | | | 11.44% | | | 88.56% |
100,000 | | | 15,350,000 | | | 11.34% | | | 88.66% |
0 | | | 15,200,000 | | | 11.24% | | | 88.76% |
-100,000 | | | 15,050,000 | | | 11.14% | | | 88.86% |
-200,000 | | | 14,900,000 | | | 11.05% | | | 88.95% |
-300,000 | | | 14,750,000 | | | 10.95% | | | 89.05% |
-400,000 | | | 14,600,000 | | | 10.85% | | | 89.15% |
-500,000 | | | 14,450,000 | | | 10.75% | | | 89.25% |
-600,000 | | | 14,300,000 | | | 10.65% | | | 89.35% |
-700,000 | | | 14,150,000 | | | 10.55% | | | 89.45% |
-800,000 | | | 14,000,000 | | | 10.45% | | | 89.55% |
-900,000 | | | 13,850,000 | | | 10.35% | | | 89.65% |
-1,000,000 | | | 13,700,000 | | | 10.25% | | | 89.75% |
-1,100,000 | | | 13,550,000 | | | 10.15% | | | 89.85% |
-1,200,000 | | | 13,400,000 | | | 10.04% | | | 89.96% |
-1,300,000 | | | 13,250,000 | | | 9.94% | | | 90.06% |
-1,400,000 | | | 13,100,000 | | | 9.84% | | | 90.16% |
-1,500,000 | | | 12,950,000 | | | 9.74% | | | 90.26% |
-1,600,000 | | | 12,800,000 | | | 9.64% | | | 90.36% |
-1,700,000 | | | 12,650,000 | | | 9.54% | | | 90.46% |
-1,800,000 | | | 12,500,000 | | | 9.43% | | | 90.57% |
-1,900,000 | | | 12,350,000 | | | 9.33% | | | 90.67% |
-2,000,000 | | | 12,200,000 | | | 9.23% | | | 90.77% |
-2,100,000 or less | | | 12,050,000 | | | 9.13% | | | 90.87% |
• | book-entry shares representing the number of whole shares of Rexahn common stock that such holder has the right to receive pursuant to the provisions of the Merger Agreement, and |
• | cash in lieu of any fractional share of Rexahn common stock. |
• | U.S. expatriates and former citizens or long-term residents of the United States; |
• | U.S. Holders whose functional currency is not the U.S. dollar; |
• | persons holding Ocuphire common stock as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment; |
• | banks, insurance companies, and other financial institutions; |
• | real estate investment trusts or regulated investment companies; |
• | brokers, dealers or traders in securities; |
• | “controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
• | S corporations, partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); |
• | persons for whom Ocuphire common stock constitutes “qualified small business stock” within the meaning of Section 1202 of the Code or as “Section 1244 stock” for purposes of Section 1244 of the Code; |
• | tax-exempt organizations or governmental organizations; |
• | persons subject to special tax accounting rules as a result of any item of gross income with respect to Ocuphire common stock being taken into account in an “applicable financial statement” (as defined in the Code); |
• | persons deemed to sell Ocuphire common stock under the constructive sale provisions of the Code; |
• | persons who hold or received Ocuphire common stock pursuant to the exercise of any employee stock option or otherwise as compensation; and |
• | tax-qualified retirement plans. |
• | an individual who is a citizen or resident of the United States; |
• | a corporation, or entity treated as a corporation, created or organized under the laws of the United States, any state thereof, or the District of Columbia; |
• | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
• | a trust that (i) is subject to the primary supervision of a U.S. court and the control of one or more “United States persons” (within the meaning of Section 7701(a)(30) of the Code) over all of its substantial decisions or (ii) has a valid election in effect to be treated as a United States person for U.S. federal income tax purposes. |
• | a U.S. Holder of shares of Ocuphire common stock will not recognize any gain or loss upon the exchange of such shares for shares of Rexahn common stock in the merger, except with respect to cash received in lieu of fractional shares (as discussed below); |
• | a U.S. Holder of shares of Ocuphire common stock will have a tax basis in the shares of Rexahn common stock received in the merger (including fractional shares deemed received and redeemed as described below) equal to the tax basis of the shares of Ocuphire common stock surrendered in exchange therefor; |
• | a U.S. Holder of shares of Ocuphire common stock will have a holding period for the shares of Rexahn common stock received in the merger (including fractional shares deemed received and redeemed as described below) that includes its holding period for its shares of Ocuphire common stock surrendered in exchange therefor; and |
• | if a U.S. Holder of shares of Ocuphire common stock acquired different blocks of shares of Ocuphire common stock at different times or at different prices, the shares of Rexahn common stock received in the merger (including fractional shares deemed received and redeemed as described below) will be allocated pro rata to each block of shares of Ocuphire common stock, and the basis and holding period of such shares of Rexahn common stock will be determined on a block-for-block approach depending on the basis and holding period of each block of shares of Ocuphire common stock exchanged for such shares of Rexahn common stock. |
• | the holder fails to furnish the holder’s taxpayer identification number, which for an individual is ordinarily his or her social security number; |
• | the holder furnishes an incorrect taxpayer identification number; |
• | the applicable withholding agent is notified by the IRS that the holder previously failed to properly report payments of interest or dividends; or |
• | the holder fails to certify under penalties of perjury that the holder has furnished a correct taxpayer identification number and that the IRS has not notified the holder that the holder is subject to backup withholding. |
• | each share of Ocuphire common stock outstanding immediately prior to the Effective Time (excluding certain shares to be canceled pursuant to the Merger Agreement and shares held by holders of Ocuphire common stock who have exercised and perfected appraisal rights or dissenters’ rights as more fully described in the section entitled “The Merger—Appraisal Rights and Dissenters’ Rights” in this proxy statement/prospectus/information statement) will automatically be converted into the right to receive a number of shares of Rexahn common stock equal to the Exchange Ratio, subject to adjustment to account for the Rexahn Reverse Stock Split and as described below (prior to the Effective Time, the outstanding Ocuphire convertible notes will be converted into Ocuphire common stock and will participate in the merger on the same basis as the other shares of Ocuphire common stock); and |
• | each option to purchase shares of Ocuphire common stock outstanding and unexercised immediately prior to the Effective Time will be assumed by Rexahn and will become an option, subject to vesting, to purchase shares of Rexahn common stock with the number of shares of Rexahn common stock underlying such options and the exercise prices for such options adjusted to reflect the Exchange Ratio and the Rexahn Reverse Stock Split. |
• | “Aggregate Valuation” means the sum of (i) the Ocuphire Valuation, plus (ii) the Rexahn Valuation. |
• | “Ocuphire Allocation Percentage” means the quotient (expressed as a percentage, with the percentage rounded to two decimal places) determined by dividing (i) the Ocuphire Valuation by (ii) the Aggregate Valuation. |
• | “Ocuphire Merger Shares” means the product determined by multiplying (i) the Post-Closing Rexahn Shares by (ii) the Ocuphire Allocation Percentage. |
• | “Ocuphire Outstanding Shares” means the total number of shares of Ocuphire capital stock outstanding immediately prior to the Effective Time expressed on a fully-diluted and as-converted to Ocuphire common stock basis, and assuming, without limitation or duplication, (i) the exercise of all Ocuphire Options outstanding as of immediately prior to the Effective Time, (ii) the conversion of all Ocuphire convertible notes and other outstanding indebtedness, (iii) the closing of the Pre-Merger Financing and (iv) the issuance of shares of Ocuphire capital stock in respect of all other outstanding options, restricted stock awards, warrants or rights to receive such shares, whether conditional or unconditional and including any outstanding options, warrants or rights triggered by or associated with the consummation of the merger (but excluding any other shares of Ocuphire common stock reserved for issuance under the Ocuphire 2018 Plan). |
• | “Ocuphire Valuation” means $120,000,000. |
• | “Rexahn Allocation Percentage” means the quotient (expressed as a percentage, with the percentage rounded to two decimal places) determined by dividing (i) the Rexahn Valuation by (ii) the Aggregate Valuation. |
• | “Rexahn Outstanding Shares” means the total number of shares of Rexahn common stock outstanding immediately prior to the Effective Time expressed on a fully-diluted basis and as converted to Rexahn common stock basis, with any in-the-money Replacement Warrants calculated based on the treasury stock method, and (i) assuming, without limitation or duplication, the exercise of all Replacement Warrants (subject to sub-clause (ii)(e) below and the settlement in shares of each in-the-money Rexahn Option outstanding as of the Effective Time solely to the extent such Rexahn Option will not be canceled at or prior to the Effective Time or exercised prior thereto, and (ii) without regard to and excluding (a) any Rexahn Options canceled at or prior to the Effective Time, (b) any out-of-the-money Rexahn Options granted under the Rexahn 2003 Plan, (c) any Rexahn Warrants that have been or will be exercised, exchanged, cancelled and/or terminated before closing, (d) any out-of-the-money Rexahn Warrants, (e) one-half of each share of Rexahn common stock underlying any out-of-the money Replacement Warrants and (f) any shares of Rexahn common stock reserved for future issuance pursuant to Rexahn stock plans. A Rexahn Option, Rexahn Warrant and Replacement Warrant is out-of-the -money if its exercise price is equivalent to or greater than $2.5025, and is in-the-money if its exercise price is less than such amount. |
• | “Rexahn Valuation” means the Rexahn Base Valuation; provided, however, to the extent that (i) the Parent Cash Amount is less than $3,200,000, then the Rexahn Base Valuation shall be reduced by $150,000 for each $100,000 that the Parent Cash Amount as so determined is less than $3,200,000, subject to a minimum Rexahn valuation of $12,000,000; and (ii) the Parent Cash Amount is greater than $6,000,000, then the Rexahn Base Valuation shall be increased by $150,000 for each $100,000 that the Parent Cash Amount as so determined is greater than $6,000,000. |
• | “Post-Closing Rexahn Shares” means the quotient determined by dividing (i) the Rexahn Outstanding Shares by (ii) the Rexahn Allocation Percentage. |
• | there must not have been issued, and remain in effect, any temporary restraining order, preliminary or permanent injunction or other order preventing the consummation of the merger or any of the other transactions contemplated by the Merger Agreement by any court of competent jurisdiction or other governmental entity of competent jurisdiction, and no law, statute, rule, regulation, ruling or decree shall be in effect which has the effect of making the consummation of the merger or any of the other transactions contemplated by the Merger Agreement illegal; |
• | receiving the “required Ocuphire stockholder approval,” whereby the holders of a majority of the outstanding shares of Ocuphire common stock must have adopted and approved the Merger Agreement and the transactions contemplated by the Merger Agreement; |
• | receiving the “required Rexahn stockholder approval,” whereby (i) the holders of a majority of the shares of Rexahn common stock outstanding on the Record Date for the Rexahn special meeting and entitled to vote thereon must have approved Proposal Nos. 2 and 3 and (ii) the holders of a majority in interest of the holders of Rexahn common stock present in person or by proxy at the Rexahn special meeting and entitled to thereon must have approved Proposal Nos. 1 and 5; |
• | the existing shares of Rexahn common stock must have been continually listed on Nasdaq through the date of the Closing, the approval of the listing of additional shares of Rexahn common stock on Nasdaq must have been obtained, and Rexahn must have caused the shares of Rexahn common stock to be approved for initial listing on Nasdaq (subject to official notice of issuance) as of the Closing; and |
• | the registration statement on Form S-4, of which this proxy statement/prospectus/information statement is a part, must have been declared effective by the SEC in accordance with the Securities Act and must not be subject to any stop order or proceeding, or any proceeding threatened by the SEC, seeking a stop order that has not been withdrawn. |
• | the representations and warranties regarding certain matters related to organization, authority, capitalization and financial advisors of the other party in the Merger Agreement must be true and correct in all material respects on the date of the Merger Agreement and on the date of the Closing with the same force and effect as if made on the date on which the merger is to be completed or, if such representations and warranties address matters as of a particular date, then as of that particular date; |
• | the remaining representations and warranties of the other party in the Merger Agreement must be true and correct on the date of the Merger Agreement and on the date of the Closing with the same force and effect as if made on the date on which the merger is to be completed or, if such representations and warranties address matters as of a particular date, then as of that particular date, except in each case, or in the aggregate, where the failure to be so true and correct would not reasonably be expected to have a Company Material Adverse Effect or Parent Material Adverse Effect (defined below), as applicable (without giving effect to any references therein to any Company Material Adverse Effect or Parent Material Adverse Effect, as applicable, or other materiality qualifications); |
• | the other party to the Merger Agreement must have performed or complied with in all material respects all of such party’s agreements and covenants required to be performed or complied with by it under the Merger Agreement at or prior to the Effective Time; and |
• | the other party must have delivered certain certificates and other documents required under the Merger Agreement for the Closing. |
• | there shall have been no change, circumstance, condition, development, effect, event, occurrence, result or state of fact that, considered together with all other such change, circumstance, financial condition, development, effect, event, occurrence, result or state of fact that have occurred prior to the applicable date of determination has or would reasonably be expected to have a material adverse effect on the business, condition, assets, liabilities or results of operations of Ocuphire or its subsidiaries, or ability to consummate the transactions contemplated by the Merger Agreement, taken as a whole (a “Company Material Adverse Effect”); provided none of the following shall be taken into account for purposes of determining whether a Company Material Adverse Effect shall have occurred: |
• | general business, economic or political conditions affecting the industries in which Ocuphire or Rexahn, as applicable, operates, except to the extent disproportionately affecting Ocuphire relative to other similarly situated companies; |
• | any natural disaster or any acts of war, armed hostilities or terrorism, except to the extent disproportionately affecting Ocuphire relative to other similarly situated companies; |
• | changes in financial, banking or securities markets, except to the extent disproportionately affecting Ocuphire relative to other similarly situated companies; |
• | any change in, or any compliance with or action taken for the purpose of complying with, applicable laws or U.S. GAAP, or interpretations thereof; |
• | the taking of any action, or failure to take any action, by Ocuphire required to comply with the terms of the Merger Agreement. |
• | Rexahn shall have received the Ocuphire lock-up agreements and Ocuphire voting agreements; |
• | The Ocuphire written consents evidencing the required Ocuphire Stockholder approval shall be in full force and effect; |
• | Rexahn shall have received (i) an original signed statement from Ocuphire that Ocuphire is not, and has not been at any time during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code, a “United States real property holding corporation,” as defined in Section 897(c)(2) of the Code, conforming to the requirements of Treasury Regulations Section 1.1445-2(c)(3) and 1.897-2(h), and (ii) an original signed notice to be delivered to the IRS in accordance with the provisions of Treasury Regulations Section 1.897-2(h)(2), together with written authorization for Rexahn to deliver such notice to the IRS on behalf of Ocuphire following the Closing, each dated as of the Closing, duly executed by an authorized officer of Ocuphire, and in form and substance reasonably acceptable to Rexahn; |
• | certain investor agreements between Ocuphire and its stockholders must have been terminated; |
• | the Pre-Merger Financing must have been consummated and Ocuphire must have received all of the proceeds of the Pre-Merger Financing (including the minimum gross proceeds of $20.0 million); |
• | No more than 5% of the holders of Ocuphire common stock shall have exercised or perfected appraisal rights in accordance with the DGCL (“Dissenting Shares); and |
• | Ocuphire must have completed the Convertible Note Conversion. |
• | there shall have been no effect, change, event, circumstance, or development, development, that, considered together with all other such effects, changes, events, circumstances, or developments, have occurred prior to the applicable date of determination has or would reasonably be expected to have a material adverse effect on the business, financial condition, assets, liabilities or results of operations of Rexahn or its subsidiaries, or ability to consummate the transactions contemplated by the Merger Agreement, taken as a whole (a “Parent Material Adverse Effect”); provided none of the following shall be taken into account for purposes of determining whether a Parent Material Adverse Effect shall have occurred: |
• | general business, economic or political conditions affecting the industry in which Rexahn operates, except to the extent disproportionately affecting Rexahn relative to other similarly situated companies; |
• | any natural disaster or any acts of war, armed hostilities or terrorism, except to the extent disproportionately affecting Rexahn relative to other similarly situated companies in the industry in which Rexahn operates; |
• | changes in financial, banking or securities markets, except to the extent disproportionately affecting Rexahn relative to other similarly situated companies in the industry in which Rexahn operates; |
• | the taking of any action required to be taken by the Merger Agreement; |
• | any change in the stock price or trading volume of Rexahn common stock (it being understood, however, that any effects, changes, events, circumstances or developments causing or contributing to any change in stock price or trading volume of Rexahn common stock may be taken into account in determining whether a Parent Material Adverse Effect has occurred, unless such effects, changes, events, circumstances or developments or otherwise are specifically excepted); |
• | any change in, or any compliance with or action taken for the purpose of complying with any law or U.S. GAAP (or interpretations of any law or U.S. GAAP); |
• | continued losses from operations or decreases in cash balances of Rexahn; and |
• | resulting from the taking of any action, or the failure to take any action, by Rexahn that is required to be taken pursuant to the Merger Agreement. |
• | the Parent Cash Amount must not be less than $0; |
• | Ocuphire shall have received the Rexahn lock-up agreements; |
• | Ocuphire must have received the resignations of each of the directors of Rexahn who are not to continue as directors of the combined company after the merger; and |
• | Rexahn must have taken all actions necessary to cause the Rexahn Board to be constituted as required by the Merger Agreement. |
• | corporate organization and power, and similar corporate matters; |
• | subsidiaries; |
• | authority to enter into the Merger Agreement and the related agreements; |
• | votes required for completion of the merger and approval of the proposals that will come before the Rexahn special meeting and that will be the subject of Ocuphire’s stockholder written consent; |
• | except as otherwise specifically disclosed pursuant to in the Merger Agreement, the fact that the consummation of the merger would not contravene or require the consent of any third-party; |
• | capitalization; |
• | financial statements and with respect to Rexahn, documents filed with the SEC and the accuracy of information contained in those documents; |
• | material changes or events; |
• | liabilities; |
• | title to assets; |
• | ownership of real property and leasehold interests; |
• | intellectual property; |
• | the existence of and validity of material contracts to which the parties or their subsidiaries are a party and any violation, default or breach to such material contracts; |
• | regulatory compliance, permits and restrictions; |
• | legal proceedings and orders; |
• | tax matters; |
• | employee and labor matters and benefit plans; |
• | environmental matters; |
• | insurance; |
• | any brokerage or finder’s fee or other fee or commission in connection with the merger; |
• | transactions with affiliates; |
• | compliance with anti-bribery laws; and |
• | with respect to Rexahn, the valid issuance in the merger of Rexahn common stock and the opinion of Oppenheimer. |
• | solicit, initiate or knowingly encourage, induce or facilitate the communication, making, submission or announcement of, any “acquisition proposal” or “acquisition inquiry” (each as defined below) or take any action that could reasonably be expected to lead to an acquisition proposal or acquisition inquiry; |
• | furnish any non-public information with respect to it to any person in connection with or in response to an acquisition proposal or acquisition inquiry; |
• | engage in discussions or negotiations with any person with respect to any acquisition proposal or acquisition inquiry; |
• | approve, endorse or recommend an acquisition proposal; |
• | execute or enter into any letter of intent or similar document or any contract contemplating or otherwise relating to any “acquisition transaction” as defined below (other than a confidentiality agreement permitted by the Merger Agreement); or |
• | publicly propose to do any of the above. |
• | any merger, consolidation, amalgamation, share exchange, business combination, issuance or acquisition of securities, reorganization, recapitalization, tender offer, exchange offer or similar transaction: (i) in which Rexahn, Ocuphire or Merger Sub is a constituent entity, (ii) in which any individual, entity, governmental entity, or “group,” as defined under applicable securities laws, directly or indirectly acquires beneficial or record ownership of securities representing more than 20% of the outstanding securities of any class of voting securities of Rexahn, Ocuphire or Merger Sub or any of their respective subsidiaries or (iii) in which Rexahn, Ocuphire or Merger Sub or any of their respective subsidiaries issues securities representing more than 20% of the outstanding securities of any class of voting securities of such party or any of its subsidiaries; or |
• | any sale, lease, exchange, transfer, license, acquisition or disposition of any business or businesses or assets that constitute or account for 20% or more of the consolidated book value or the fair market value of the assets of Rexahn, Ocuphire or Merger Sub and their respective subsidiaries, as applicable, taken as a whole. |
• | neither such party nor any representative of such party has materially breached the solicitation provisions of the Merger Agreement described above; |
• | such party’s board of directors concludes in good faith, based on the advice of outside legal counsel, that the failure to take such action is reasonably likely to be inconsistent with the fiduciary duties of such board of directors under applicable legal requirements; |
• | such party receives from the third party an executed confidentiality agreement containing provisions at least as favorable to such party as those contained in the confidentiality agreement between Rexahn and Ocuphire, or the third party is already party to a confidentiality agreement that is still in effect and contains provisions that require any counterparty thereto (and any of its affiliates and representatives) that receives material nonpublic information of or with respect to Rexahn or the Company to keep such information confidential; and |
• | prior to or substantially contemporaneously with furnishing any non-public information to such third-party, such party furnishes the same non-public information to the other party to the extent not previously furnished. |
• | the Rexahn Board or the Ocuphire Board, as applicable, determines in good faith (after consultation with its outside legal counsel) that the failure to make a change in recommendation in response to such superior offer would reasonably be expected to be inconsistent with the fiduciary duties of such board of directors under applicable legal requirements; |
• | Rexahn or Ocuphire, as applicable, has given the other party prior written notice of its intention to consider making a change in recommendation at least three business days prior to making any such change in recommendation, such notice is referred to in this proxy statement/prospectus/information statement as a “determination notice”; |
• | the party delivering the determination notice has provided the other party a summary of the material terms and conditions of the acquisition proposal constituted a superior offer; |
• | the party delivering the determination notice has given the other party three business days after the delivery of the determination notice to propose revisions to the terms of the Merger Agreement or to make another proposal and has made its representatives reasonably available to negotiate in good faith with respect to such proposed revisions or other proposal; and |
• | after considering the results of any such negotiations and given effect to the proposals made by the other party, if any, and after consultation with outside legal counsel, the Rexahn Board or the Ocuphire Board, as applicable, determines in good faith that the third-party acquisition proposal still constitutes a superior offer and that the failure to make a change in recommendation in respect thereof would reasonably be expected to be inconsistent with the fiduciary duties of such board of directors under applicable legal requirements. |
• | the Rexahn Board or the Ocuphire Board, as applicable, determines in good faith (after consultation with its outside legal counsel) that the failure to make a change in recommendation in response to such change in circumstance would reasonably be expected to be inconsistent with the fiduciary duties of such board of directors under applicable legal requirements; |
• | Rexahn or Ocuphire, as applicable, has given the other party a determination notice at least three business days prior to making any such change in recommendation; |
• | the party delivering the determination notice has provided the other party a reasonably detailed description of the change in circumstance; |
• | the party delivering the determination notice has given the other party three business days after the delivery of the determination notice to propose revisions to the terms of the Merger Agreement or to make another proposal and has made its representatives reasonably available to negotiate in good faith with respect to such proposed revisions or other proposal; and |
• | after considering the results of any such negotiations and given effect to the proposals made by the other party, if any, and after consultation with outside legal counsel, the Rexahn Board or the Ocuphire Board, as applicable, determines in good faith that the failure to make a change in recommendation in response to the change in circumstance would reasonably be expected to be inconsistent with the fiduciary duties of such board of directors under applicable legal requirements. |
• | declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of capital stock or repurchase, redeem or otherwise reacquire any shares of capital stock or other securities (except in connection with the payment of the exercise price and/or withholding taxes incurred upon the exercise, settlement or vesting of any award granted under a Rexahn employee benefit plan in accordance with the terms of such award in effect on the date of the Merger Agreement); |
• | sell, issue, grant, pledge, or otherwise dispose of or encumber or authorize any of the foregoing with respect to: any capital stock or other security (except for Rexahn common stock issued upon the valid exercise of outstanding options or warrants to purchase shares of Rexahn common stock); any option, warrant or right to acquire any capital stock or any other security; or any instrument convertible into or exchangeable for any capital stock or other security of Rexahn; |
• | except as required to give effect to anything in contemplation of the Closing, amend the certificate of incorporation, bylaws or other charter or organizational documents of Rexahn, or effect or be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split, or similar transaction except as related to the proposed transactions under the Merger Agreement; |
• | form any subsidiary or acquire any equity interest, or other interest in any other entity, or enter into any joint venture with any other entity; |
• | lend money to any person (except for the advancement of reasonable expenses to employees, directors and consultants in the ordinary course of business); incur or guarantee any indebtedness for borrowed money; guarantee any debt securities of others; or make any capital expenditure or capital commitment in excess of the amounts set forth in Rexahn’s operating budget delivered to Ocuphire concurrently with the Merger Agreement; |
• | other than as required by law or the terms of a Rexahn employee plan in effect as of the date of the Merger Agreement, adopt, terminate, establish or enter into any Rexahn employee plan; cause or permit any Rexahn employee plan to be amended in any material respect; pay any bonus or make any profit-sharing or similar payment to, or increase the amount of the wages, salary, commissions, fringe benefits or other compensation or remuneration payable to, any of its employees, officers or directors (other than in the ordinary course of business consistent with past practice); or increase the severance, retention or change of control benefits offered to any current, former or new employees, directors or consultants; |
• | recognize any labor union, labor organization, or similar entity except as otherwise required by law and after advance notice to Ocuphire; |
• | acquire any material asset or sell, lease or otherwise irrevocably dispose of any of its material assets or properties, or grant any encumbrance with respect to such assets or properties; |
• | make, change or revoke any material tax election, fail to pay any income or other material tax as such tax becomes due and payable, file any material amended tax return, settle or compromise any income or other material tax liability, enter into any tax allocation, sharing, indemnification or other similar agreement or arrangement (other than customary commercial contracts entered into in the ordinary course of business the principal subject matter of which is not taxes), request or consent to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material taxes (other than pursuant to an extension of time to file any tax return granted in the ordinary course of business of not more than six months), or adopt or change any material accounting method in respect of taxes; |
• | enter into, materially amend or terminate certain material contracts; |
• | other than the incurrence or payment of certain transaction expenses, make any expenditures, incur any liabilities or discharge or satisfy any liabilities, in each case, outside of the ordinary course of business; |
• | other than as required by law or U.S. GAAP, take any action to change accounting policies or procedures; |
• | initiate or settle any legal proceeding; or |
• | agree, resolve, or commit to do any of the foregoing. |
• | declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of its capital stock of Ocuphire or repurchase, redeem or otherwise reacquire any shares of capital stock or other securities; |
• | sell, issue, grant, pledge, or otherwise dispose of or encumber or authorize any of the foregoing actions with respect to: any capital stock or other security of Ocuphire or any of its subsidiaries (except for shares of Ocuphire common stock issued upon the valid exercise of Ocuphire options); any option, warrant or right to acquire any capital stock or any other security, other than option grants to employees and service providers in the ordinary course of business; or any other instrument convertible into or exchangeable for any capital stock or any other security of Ocuphire or its subsidiaries; |
• | except as required to give effect to anything in contemplation of the Closing, amend the certificate of incorporation, bylaws or other charter or organizational documents of Ocuphire or its subsidiaries, or effect or become a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split, liquidation, dissolution or similar transaction except as related to the proposed transactions under the Merger Agreement; |
• | form any subsidiary or acquire any equity interest, or other interest in any other entity or enter into a joint venture with any other entity or enter into a new line of business; |
• | lend money to any person (except for the advancement of expenses to employees, directors and consultants in the ordinary course of business); incur or guarantee any indebtedness for borrowed money; guarantee any debt securities of others; or make any capital expenditure or capital commitment in excess of the budgeted capital expenditure and commitment amounts set forth in the Ocuphire operating budget delivered to Rexahn concurrently with the execution of this Merger Agreement; |
• | other than as required by applicable law or the terms of any Ocuphire employee benefit plan: adopt, terminate, establish or enter into any employee plan; cause or permit any employee plan to be amended in any material respect; pay any bonus or make any profit-sharing or similar payment to, or increase the amount of the wages, salary, commissions, benefits or other compensation or remuneration payable to, any of its directors, officers or employees, other than increases in base salary and special cash bonus opportunities and payments made in the ordinary course of business consistent with past practice; or increase the severance, retention or change of control benefits offered to any current, former or new employees, directors or consultants; |
• | recognize any labor union, labor organization, or similar entity except as otherwise required by law and after advance notice to Rexahn; |
• | acquire any material asset or sell, lease or otherwise irrevocably dispose of any of its material assets or properties, or grant any encumbrance with respect to such assets or properties; |
• | sell, assign, transfer, license, sublicense, abandon, permit to lapse or otherwise dispose of any material Ocuphire intellectual property rights (other than pursuant to non-exclusive licenses in the ordinary course of business); |
• | make, change or revoke any material tax election, fail to pay any income or other material tax as such tax becomes due and payable, file any material amended tax return, settle or compromise any income or other material tax liability, enter into any tax allocation, sharing, indemnification or other similar agreement or arrangement (other than customary commercial contracts entered into in the ordinary course of business the principal subject matter of which is not taxes), request or consent to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material taxes (other than pursuant to an extension of time to file any tax return granted in the ordinary course of business of not more than six months), or adopt or change any material accounting method in respect of taxes; |
• | enter into any Ocuphire material contract outside the ordinary course of business, or materially amend or terminate certain material contracts; |
• | other than the incurrence or payment of certain transaction expenses, make any expenditures, incur any liabilities or discharge or satisfy any liabilities, in each case, outside of the ordinary course of business; |
• | other than as required by law or U.S. GAAP, take any action to change accounting policies or procedures; |
• | initiate or settle any legal proceeding; or |
• | agree, resolve, or commit to do any of the foregoing. |
• | file or otherwise submit all applications and notices required to be filed in connection with the merger and the other transactions contemplated by the Merger Agreement; |
• | use commercially reasonable efforts to obtain each consent reasonably required to be obtained in connection with the merger and the other transactions contemplated by the Merger Agreement; |
• | use commercially reasonable efforts to lift any injunction prohibiting, or any other legal bar to, the merger or the other transactions contemplated by the Merger Agreement; and |
• | use commercially reasonable efforts to satisfy the conditions precedent to the consummation of the transactions contemplated by the Merger Agreement. |
• | Rexahn will use its commercially reasonable efforts to (i) maintain the listing of its common stock on Nasdaq until the Closing and to obtain approval for listing of the combined company on Nasdaq and (ii) to the extent required by the rules and regulations of Nasdaq, to prepare and submit to Nasdaq a notification form for the listing of the shares of Rexahn common stock to be issued in connection with the merger and to cause such shares to be approved for listing (subject to official notice of issuance); (iii) to effect the Rexahn Reverse Stock Split; and (iv) to the extent required by Nasdaq Marketplace Rule 5110, to file an initial listing application for Rexahn common stock on Nasdaq and to cause such listing application to be conditionally approved prior to the Effective Time; |
• | for a period of six years after the Closing, Rexahn will indemnify each of the current and former directors and officers of Rexahn and Ocuphire to the fullest extent permitted under the DGCL and will maintain directors’ and officers’ liability insurance for the directors and officers of Rexahn and Ocuphire; |
• | Rexahn shall maintain directors’ and officers’ liability insurance policies commencing at the Closing, on commercially reasonable terms and conditions and with coverage limits customary for U.S. public companies similarly situated to Rexahn, and shall purchase a six-year prepared “tail” insurance policy for the non-cancellable extension of the directors’ and officers’ liability coverage of Rexahn’s existing directors’ and officers’ insurance policies with respect to any claim related to any period of time at or prior to the Effective Time of the merger; and |
• | Ocuphire shall use reasonable best efforts to cause to be taken all actions necessary to consummate the Pre-Merger Financing prior to the Closing. |
• | by mutual written consent of Rexahn and Ocuphire; |
• | by either Rexahn or Ocuphire if the transactions contemplated by the Merger Agreement shall not have been consummated by November 14, 2020 (the “End Date”); provided, however, that this right to terminate the Merger Agreement will not be available to any party whose action or failure to act has been a principal cause of the failure of the merger to occur on or before the End Date and such action or failure to act constitutes a breach of the Merger Agreement; provided, further, that the End Date shall be extended by 60 days upon request of either party if a request for additional information has been made by any government authority, or in the event that the SEC has not declared effective the registration statement on Form S-4 by the date that is 60 days prior to the End Date, of which this |
• | by either Rexahn or Ocuphire if a court of competent jurisdiction or governmental entity has issued a final and no appealable order, decree or ruling or taken any other action that has the effect of permanently restraining, enjoining or otherwise prohibiting the merger or any of the other transactions contemplated by the Merger Agreement; |
• | by Rexahn if the required Ocuphire stockholder approval has not been obtained within 5 business days of the registration statement on Form S-4, of which this proxy statement/prospectus/information statement is a part, becoming effective; provided that this right to terminate the Merger Agreement will not be available to Rexahn once Ocuphire obtains such stockholder approval; |
• | by either Rexahn or Ocuphire if the Rexahn special meeting shall have been held and completed and Rexahn Stockholders shall have taken a final vote and shall not have approved Proposal Nos. 1, 2, 3 and 5; provided, that Rexahn may not terminate the Merger Agreement pursuant to this provision if the failure to obtain the required Rexahn Stockholder approval was directly caused by the action or failure to act of Rexahn and such action or failure to act constitutes a material breach by Rexahn of the Merger Agreement; |
• | by Ocuphire, at any time prior to receiving the required Rexahn Stockholder approval, if any of the following circumstances shall occur (each of the following, a “Rexahn triggering event”): |
• | The Rexahn Board fails to recommend that the Rexahn Stockholders vote to approve Proposal Nos. 1, 2, 3, 4 or 5 or makes a change in recommendation; |
• | The Rexahn Board, or any committee thereof, publicly approves, endorses or recommends any acquisition proposal; |
• | Rexahn enters into any letter of intent or similar document or any contract relating to any acquisition proposal, other than a confidentiality agreement permitted pursuant to the Merger Agreement; or |
• | Rexahn, or any director or officer of Rexahn, has willfully and intentionally breached the no solicitation provisions set forth in the Merger Agreement and described above in the section entitled “- No Solicitation”; |
• | by Rexahn, at any time prior to receiving the required Ocuphire Stockholder approval, if any of the following circumstances shall occur (each an “Ocuphire triggering event”): |
• | The Ocuphire Board fails to recommend that Ocuphire Stockholders vote to adopt the Merger Agreement, thereby approving the merger, or makes a change in recommendation; |
• | The Ocuphire Board, or any committee thereof, publicly approves, endorses or recommends any acquisition proposal; or |
• | Ocuphire enters into any letter of intent or similar document or any contract relating to any acquisition proposal (other than a confidentiality agreement permitted pursuant to the Merger Agreement); or |
• | Ocuphire, or any director or officer of Ocuphire, has willfully and intentionally breached the no solicitation provisions set forth in the Merger Agreement and described above in the section titled “– No Solicitation”; |
• | by Rexahn or Ocuphire if the other party has breached any of its representations, warranties, covenants or agreements contained in the Merger Agreement or if any representation or warranty of the other party has become inaccurate, in either case such that the conditions to the Closing would not be |
• | by Rexahn, at any time, upon Rexahn entering into a definitive agreement to effect a superior offer if (i) Rexahn has received a superior offer, (ii) Rexahn has complied with its obligations under the change in recommendation provisions of the Merger Agreement as described in the section titled “– Change in Board Recommendation,” and (iii) within five business days of termination, Rexahn pays the applicable termination fee described below. |
• | if (i) the Merger Agreement is terminated by Rexahn or Ocuphire if the merger is not consummated by the End Date, subject to the conditions described above; (ii) an acquisition proposal with respect to Rexahn was publicly announced or disclosure or otherwise communicated to Rexahn or the Rexahn Board after the date of the Merger Agreement but prior to the termination of the Merger Agreement, and (iii) within six months after the date of such termination, Rexahn enters into a definitive agreement for or consummates a subsequent transaction (with all references to 50% in the definition of acquisition transaction being treated as references to 20%); |
• | if (i) the Merger Agreement is terminated by either Rexahn or Ocuphire if the Rexahn special meeting shall have been held and completed, and Rexahn Stockholders shall have not approved Proposal Nos. 1, 2, 3 or 5; (ii) an acquisition proposal with respect to Rexahn was publicly announced or disclosure or otherwise communicated to Rexahn or the Rexahn Board after the date of the Merger Agreement but prior to the termination of the Merger Agreement, and (iii) within six months after the date of such termination, Rexahn enters into a definitive agreement for or consummates a subsequent transaction (with all references to 50% in the definition of acquisition transaction being treated as references to 20%); |
• | if (i) the Merger Agreement is terminated by Ocuphire if a Rexahn triggering event has occurred; (ii) an acquisition proposal with respect to Rexahn was publicly announced or disclosure or otherwise communicated to Rexahn or the Rexahn Board after the date of the Merger Agreement but prior to the termination of the Merger Agreement, and (iii) within six months after the date of such termination, Rexahn enters into a definitive agreement for or consummates a subsequent transaction (with all references to 50% in the definition of acquisition transaction being treated as references to 20%); |
• | if (i) the Merger Agreement is terminated by Ocuphire if Rexahn or Merger Sub has breached any of its representations, warranties, covenants or agreements contained in the Merger Agreement or if any representation or warranty of Rexahn or Merger Sub has become inaccurate, in either case such that the conditions to the Closing would not be satisfied as of the time of such breach or inaccuracy, subject to a 15-day cure period; (ii) an acquisition proposal with respect to Rexahn was publicly announced or disclosure or otherwise communicated to Rexahn or the Rexahn Board after the date of the Merger Agreement but prior to the termination of the Merger Agreement, and (iii) within six months after the date of such termination, Rexahn enters into a definitive agreement for or consummates a subsequent transaction (with all references to 50% in the definition of acquisition transaction being treated as references to 20%); or |
• | if the Merger Agreement is terminated by Rexahn to enter into a definitive agreement to effect a superior offer. |
• | if (i) the Merger Agreement is terminated by Ocuphire or Rexahn if the merger is not consummated by the End Date, subject to the conditions described above; (ii) an acquisition proposal with respect to |
• | if (i) the Merger Agreement is terminated by Rexahn if the required Ocuphire Stockholder approval has not been obtained within 5 business days of the registration statement on Form S-4, of which this proxy statement/prospectus/information statement is a part, becoming effective; (ii) an acquisition proposal with respect to Ocuphire was publicly announced or disclosure or otherwise communicated to Ocuphire or the Ocuphire Board after the date of the Merger Agreement but prior to the termination of the Merger Agreement, and (iii) within six months after the date of such termination, Ocuphire enters into a definitive agreement for or consummates a subsequent transaction (with all references to 50% in the definition of acquisition transaction being treated as references to 20%); |
• | if (i) the Merger Agreement is terminated by Rexahn if an Ocuphire triggering event has occurred; (ii) an acquisition proposal with respect to Ocuphire was publicly announced or disclosure or otherwise communicated to Ocuphire or the Ocuphire Board after the date of the Merger Agreement but prior to the termination of the Merger Agreement, and (iii) within six months after the date of such termination, Ocuphire enters into a definitive agreement for or consummates a subsequent transaction (with all references to 50% in the definition of acquisition transaction being treated as references to 20%); or |
• | if (i) the Merger Agreement is terminated by Rexahn if Ocuphire has breached any of its representations, warranties, covenants or agreements contained in the Merger Agreement or if any representation or warranty of Ocuphire has become inaccurate, in either case such that the conditions to the Closing would not be satisfied as of the time of such breach or inaccuracy, subject to a 15-day cure period; (ii) an acquisition proposal with respect to Ocuphire was publicly announced or disclosure or otherwise communicated to Ocuphire or the Ocuphire Board after the date of the Merger Agreement but prior to the termination of the Merger Agreement, and (iii) within six months after the date of such termination, Ocuphire enters into a definitive agreement for or consummates a subsequent transaction (with all references to 50% in the definition of acquisition transaction being treated as references to 20%). |
• | 90% of all payments received by Rexahn or its affiliates during such CVR Payment Period from or on behalf of BioSense pursuant to the BioSense Agreement, minus the amount of any fees, costs or expenses paid by Rexahn and its affiliates during such CVR Payment Period related to the performance of Rexahn’s obligations under the BioSense Agreement or incurred by Rexahn and its affiliates in connection with enforcing Rexahn’s rights under the BioSense Agreement; |
• | 90% of all payments received by Rexahn or its affiliates during such CVR Payment Period from or on behalf of HaiChang pursuant to the HaiChang Agreement, minus the amount of any fees, costs or expenses paid by Rexahn and its affiliates during such CVR Payment Period related to the performance of Rexahn’s obligations under the HaiChang Agreement or incurred by Rexahn and its affiliates in connection with enforcing Rexahn’s rights under the HaiChang Agreement; and |
• | 75% of (a) all cash consideration paid by a third party to Rexahn or its affiliates during the applicable CVR Payment Period in connection with the grant, sale or transfer of rights to Rexahn’s pre-Closing intellectual property (“Parent IP”) (other than a grant, sale or transfer of rights involving a sale or disposition of the post-merger combined company) that is entered into during the 10-year period after the Closing (a “Parent IP Deal”); plus (b) with respect to any non-cash consideration received by Rexahn or its affiliates from a third party during the applicable CVR Payment Period in connection with any Parent IP Deal, all amounts received by Rexahn and its affiliates for such non-cash consideration at the time such non-cash consideration is monetized by Rexahn or its affiliates; minus (c) any Permitted Parent IP Deductions (as defined below) during such CVR Payment Period. |
• | U.S. expatriates and former citizens or long-term residents of the United States; |
• | Rexahn U.S. Holders whose functional currency is not the U.S. dollar; |
• | persons holding Rexahn common stock as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment; |
• | banks, insurance companies, and other financial institutions; |
• | real estate investment trusts or regulated investment companies; |
• | brokers, dealers or traders in securities; |
• | “controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
• | S corporations, partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); |
• | persons for whom Rexahn common stock constitutes “qualified small business stock” within the meaning of Section 1202 of the Code or “Section 1244 stock” for purposes of Section 1244 of the Code; |
• | tax-exempt organizations or governmental organizations; |
• | persons subject to special tax accounting rules as a result of any item of gross income with respect to Rexahn common stock being taken into account in an “applicable financial statement” (as defined in the Code); |
• | persons deemed to sell Rexahn common stock under the constructive sale provisions of the Code; |
• | persons who hold or received Rexahn common stock pursuant to the exercise of any employee stock option or otherwise as compensation; and |
• | tax-qualified retirement plans. |
• | an individual who is a citizen or resident of the United States; |
• | a corporation created or organized under the laws of the United States, any state thereof, or the District of Columbia; |
• | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
• | a trust that (i) is subject to the primary supervision of a U.S. court and the control of one or more “United States persons” (within the meaning of Section 7701(a)(30) of the Code) over all of its substantial decisions or (ii) has a valid election in effect to be treated as a United States person for U.S. federal income tax purposes. |
• | “Conversion Price” means the per share price resulting from the quotient of (1) $100,000,000 less the aggregate amount of One Hundred Seventy-Five Percent (175%) times the Note Value of all of the convertible notes divided by (2) the Fully Diluted Shares (as defined below). |
• | “Fully Diluted Shares” means as of the Conversion Date the sum of the following: (1) all of the issued outstanding shares of Ocuphire common stock; and (2) the aggregate number of shares of Ocuphire common stock reserved for issuance under all outstanding options or other awards under equity incentive plans of Ocuphire in effect as of the Conversion Date. |
• | “Note Value” means the outstanding principal balance of, plus the accrued but unpaid interest on, each convertible note. |
• | The Rexahn Reverse Stock Split may be necessary to increase Rexahn’s stock price to meet Nasdaq’s minimum bid price requirement upon the Closing; |
• | the Rexahn Board believes effecting the Rexahn Reverse Stock Split may be an effective means of avoiding a delisting of Rexahn common stock from Nasdaq in the future; |
• | the Rexahn Reverse Stock Split may be required in order to make sufficient shares of Rexahn common stock available for issuance to Ocuphire Stockholders pursuant to the Merger Agreement and the Investor Warrants; |
• | the Rexahn Board believes a higher stock price may help generate investor interest in Rexahn and help Rexahn attract and retain employees; and |
• | If the Rexahn Reverse Stock Split successfully increases the per share price of Rexahn common stock, the Rexahn Board believes this increase may increase trading volume in Rexahn common stock and facilitate future financings by Rexahn. |
• | the historical trading price and trading volume of the Rexahn common stock; |
• | the then-prevailing trading price and trading volume of the Rexahn common stock and the expected impact of the reverse stock split on the trading market for Rexahn common stock in the short- and long-term; |
• | the ability of Rexahn to continue its listing on the Nasdaq Capital Market; |
• | which reverse stock split ratio would result in the least administrative cost to Rexahn; and |
• | prevailing general market and economic conditions. |
• | The number of outstanding shares of Rexahn common stock will be reduced and each Rexahn Stockholder will own fewer shares than they currently own. |
• | The number of shares of Rexahn common stock reserved and available for issuance under Rexahn’s equity-based compensation plans and the number of shares of Rexahn common stock issuable upon exercise of outstanding options and warrants will be reduced proportionately based on the reverse stock split ratio selected by the Rexahn Board, and the exercise price of all outstanding options and warrants will be increased proportionately. |
• | Except for adjustments that may result from the treatment of fractional shares resulting from the Rexahn Reverse Stock Split, which are explained below under the section entitled “—Fractional Shares,” each stockholder will hold the same percentage of Rexahn common stock immediately following the Rexahn Reverse Stock Split as the stockholder held immediately prior to the Rexahn Reverse Stock Split. |
• | The voting rights, rights to dividends and distributions and other rights of Rexahn common stock will not be changed as a result of the Rexahn Reverse Stock Split. |
• | The Rexahn Reverse Stock Split will not affect the number of authorized shares of Rexahn common stock or preferred stock which will continue to be authorized pursuant to the Rexahn Certificate of Incorporation, or the par value of Rexahn common stock or preferred stock. As described further below, because the number of authorized shares will not be reduced proportionately, the Rexahn Reverse Stock Split will increase the Rexahn Board’s ability to issue authorized and unissued shares without further stockholder action. |
| | | Shares Issued and Outstanding(1) | | | Shares Authorized and Reserved for Issuance(1)(2) | | | Shares Authorized and Unreserved for Issuance(1) | | | Total Authorized(1) | |
As of September 10, 2020 | | | 4,483,198 | | | 1,211,192 | | | 69,305,610 | | | 75,000,000 |
1-for-3 Reverse Split | | | 1,494,399 | | | 403,730 | | | 73,101,871 | | | 75,000,000 |
1-for-4 Reverse Split | | | 1,120,799 | | | 302,798 | | | 73,576,403 | | | 75,000,000 |
1-for-5 Reverse Split | | | 896,639 | | | 242,238 | | | 73,861,123 | | | 75,000,000 |
(1) | These estimates do not reflect the potential effects of cashing out of fractional shares that may result from the reverse stock split. |
(2) | Includes warrants to purchase 925,732 shares of Rexahn common stock with a weighted average exercise price of $21.52 per share, options to purchase 146,224 shares of Rexahn common stock with a weighted average exercise price of $24.06 per share and 139,236 shares reserved for future issuance under the Rexahn 2013 Plan. Does not include any shares of Rexahn common stock issuable upon the exercise or conversion of securities that may have been issued since September 10, 2020 or any shares of Rexahn common stock reserved or to be reserved in connection with the Pre-Merger Financing. |
• | the market price per share of Rexahn common stock after the Rexahn Reverse Stock Split will rise in proportion to the reduction in the number of shares of Rexahn common stock outstanding before the Rexahn Reverse Stock Split; |
• | the Rexahn Reverse Stock Split will result in a per share price that will attract brokers and investors who do not trade in lower priced stocks; |
• | the Rexahn Reverse Stock Split will result in a per share price that will increase the ability of Rexahn to attract and retain employees; |
• | the bid price per share will either exceed or remain in excess of the $1.00 minimum bid price as required by Nasdaq for continued listing; or |
• | that Rexahn will otherwise meet the requirements of Nasdaq for initial listing on the Nasdaq Capital Market, including the $4.00 minimum bid price upon the Closing. |
• | U.S. expatriates and former citizens or long-term residents of the United States; |
• | Rexahn U.S. Holders whose functional currency is not the U.S. dollar; |
• | persons holding Rexahn common stock as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment; |
• | banks, insurance companies, and other financial institutions; |
• | real estate investment trusts or regulated investment companies; |
• | brokers, dealers or traders in securities; |
• | persons for whom Rexahn common stock constitutes “qualified small business stock” within the meaning of Section 1202 of the Code; |
• | “controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
• | S corporations, partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); |
• | tax-exempt organizations or governmental organizations; |
• | persons subject to special tax accounting rules as a result of any item of gross income with respect to Rexahn common stock being taken into account in an “applicable financial statement” (as defined in the Code); |
• | persons deemed to sell Rexahn common stock under the constructive sale provisions of the Code; |
• | persons who hold or received Rexahn common stock pursuant to the exercise of any employee stock option or otherwise as compensation; and |
• | tax-qualified retirement plans. |
• | the holder fails to furnish the holder’s taxpayer identification number, which for an individual is ordinarily his or her social security number; |
• | the holder furnishes an incorrect taxpayer identification number; |
• | the applicable withholding agent is notified by the IRS that the holder previously failed to properly report payments of interest or dividends; or |
• | the holder fails to certify under penalties of perjury that the holder has furnished a correct taxpayer identification number and that the IRS has not notified the holder that the holder is subject to backup withholding. |
• | RX-3117 is a novel, investigational oral, small molecule nucleoside compound. Once intracellularly activated (phosphorylated) by the enzyme UCK2, it is incorporated into the DNA or RNA of cells and inhibits both DNA and RNA synthesis, which induces apoptotic cell death of tumor cells. RX-3117 has been the subject of a Phase 2a clinical trial in combination with Celgene’s ABRAXANE® (paclitaxel protein-bound particles for injectable suspension) as a first-line treatment in patients newly diagnosed with metastatic pancreatic cancer. The trial reached its target enrollment in February 2019. As of July 24, 2019, an overall response rate of 23% had been observed in 40 patients that had at least one scan on treatment. Preliminary and unaudited data indicates that the median progression free survival for patients in the study is approximately 5.4 months. Complete data from the trial is expected to be available in 2020. Rexahn does not plan to conduct or sponsor any additional trials with RX-3117. |
• | RX-5902 is a potential small molecule modulator of the Wnt/beta-catenin pathway which plays a key role in cancer cell proliferation and tumor growth. In August 2018, Rexahn entered into a Clinical Trial Collaboration and Supply Agreement (the “Collaboration Agreement”) with Merck Sharp & Dohme B.V. (“Merck”) to evaluate the combination of RX-5902 and Merck’s anti-PD-1 therapy, KEYTRUDA® (pembrolizumab) in a Phase 2 trial in patients with metastatic triple negative breast cancer (“TNBC”). On April 7, 2020, Rexahn notified Merck that it was terminating the Collaboration Agreement, effective immediately, in connection with Rexahn’s determination to discontinue development of RX-5902 for the treatment of TNBC. Rexahn does not plan to conduct or sponsor any additional trials with RX-5902. |
• | RX-0301 is a potential potent inhibitor of the synthesis of the protein kinase Akt-1, which Rexahn believes plays a critical role in cancer cell proliferation, survival, angiogenesis, metastasis, and drug resistance. RX-0301 is currently in preclinical development by HaiChang as a nano-liposomal formulation of RX-0201 (Archexin®) using HaiChang’s proprietary QTsome™ technology. On February 8, 2020, Rexahn entered into the HaiChang Agreement, pursuant to which Rexahn granted HaiChang an exclusive (even as to Rexahn), royalty-bearing, sublicensable worldwide license to research, develop and commercialize RX-0201 and RX-0301. The HaiChang Agreement supersedes a prior agreement with HaiChang to develop RX-0301 under which HaiChang was to conduct certain preclinical and clinical activities through completion of a Phase 2a proof-of-concept clinical trial in hepatocellular carcinoma (“HCC”). |
• | developing drugs; |
• | undertaking preclinical testing and human clinical trials; |
• | obtaining FDA and other regulatory approvals of drugs; |
• | formulating and manufacturing drugs; and |
• | launching, marketing and selling drugs. |
• | The Anti-Kickback Statute, which prohibits, among other things, knowingly or willingly offering, paying, soliciting or receiving remuneration (interpreted to include anything of value), directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of, any health care items or service for which payment may be made, in whole or in part, by federal healthcare programs such as Medicare and Medicaid. This statute has been interpreted to apply to arrangements between pharmaceutical companies on one hand and prescribers, purchasers and formulary managers on the other. Liability may be established under the federal Anti-Kickback Law without proving actual knowledge of the statute or specific intent to violate it. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Law constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. Although there are a number of statutory exemptions and regulatory safe harbors to the federal Anti-Kickback Law protecting certain common business arrangements and activities from prosecution or regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exemption or safe harbor, or for which no exception or safe harbor is available, may be subject to scrutiny. |
• | The FCA, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment of government funds or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly concealing or knowingly and improperly avoiding, decreasing or concealing an obligation to pay money to the federal government. Private individuals, commonly known as “whistleblowers,” can bring civil FCA qui tam actions, on behalf of the government and such individuals and may share in amounts paid by the entity to the government in recovery or settlement. FCA liability is potentially significant in the healthcare industry because the statute provides for treble damages and significant mandatory penalties per false or fraudulent claim or statement. The government may assert that a claim including items or services resulting from a violation of the Anti-Kickback Statute constitutes a false or fraudulent claim under the federal civil FCA. Many pharmaceutical and other healthcare companies have been investigated and have reached substantial financial settlements with the federal government under the FCA for a variety of alleged improper marketing activities, including: providing free product to customers with the expectation that the customers would bill federal programs for the product; providing sham consulting fees, grants, free travel and other benefits to physicians to induce them to prescribe the company’s products; and inflating prices reported to private price publication services, which are used to set drug payment rates under government healthcare programs. In addition, in recent years the government has pursued civil FCA cases against a number of pharmaceutical companies for causing false claims to be submitted as a result of the marketing of their products for unapproved, and thus non-reimbursable, uses. Pharmaceutical and other healthcare companies also are subject to other federal false claim laws, including, among others, federal criminal healthcare fraud and false statement statutes that extend to non-government health benefit programs. |
• | Analogous state and local laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; state and foreign laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; and state and foreign laws that require drug manufacturers to report information related to clinical trials, or information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; |
• | The Physician Payment Sunshine Act, being implemented as the Open Payments Program, which requires manufacturers of drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain |
• | The FCPA and other similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from providing money or anything of value to officials of foreign governments, foreign political parties or international organizations with the intent to obtain or retain business or seek a business advantage. Recently, there has been a substantial increase in anti-bribery law enforcement activity by U.S. regulators, with more frequent and aggressive investigations and enforcement proceedings by both the Department of Justice and the SEC. Violations of U.S. or foreign laws or regulations could result in the imposition of substantial fines, interruptions of business, loss of supplier, vendor or other third-party relationships, termination of necessary licenses and permits and other legal or equitable sanctions. Other internal or government investigations or legal or regulatory proceedings, including lawsuits brought by private litigants, may also follow as a consequence. |

• | Reduction in pupil diameter with durable effects. In multiple Phase 2 trials Nyxol reduced pupil diameter by approximately 20% (~1 – 1.5 mm) in both mesopic (dim) and photopic (bright) conditions, with such reductions sustained over 24 hours. |
• | Improvement in low contrast visual acuity. When studied in patients with NVD in multiple Phase 2 trials, Nyxol showed statistically significant improvement in low contrast mesopic best-corrected distance visual acuity at ≥1 and ≥2 lines, with a trend at ≥3 lines on a standard visual chart. |
• | Promising tolerability profile. To date, Nyxol has been observed to be well tolerated, with unchanged or decreased intraocular pressure, in the 7 completed Phase 1 and Phase 2 clinical trials conducted. Nyxol produces a transient, mild hyperemia effect that disappears within 4 to 8 hours or immediately upon application of anti-redness eye drops. Nyxol is also observed to have no systemic effects, such as changes in blood pressure or heart rate. |
• | Designed to be a convenient, once-daily eye drop. Nyxol is being evaluated for chronic use as a once-daily administration before bedtime. Nyxol has also been shown in multiple Phase 2 trials to have an over 24-hour durable effect, which could allow for better patient compliance. |
• | Stable, cost-effective ophthalmic formulation. Nyxol is a single-use, preservative-free, proprietary eye drop formulation with good stability for eventual commercialization. Its active pharmaceutical ingredient, phentolamine mesylate USP grade, is a small molecule with advantages of standardized, scalable, lower-cost manufacturing processes. |
• | NVD, a condition in which peripheral imperfections (aberrations) of the cornea scatter light when the pupil opens wide in dim light. Patients with NVD experience glare, halos, starbursts, and decreased contrast sensitivity. NVD is a new indication with no approved therapies. |
• | RM, a reversal of pharmacologically induced dilation of the pupils, where dilation leads to increased sensitivity to light and an inability to focus, making it difficult to read, work, and drive. RM is a single-use indication with no commercially available therapies. |
• | Presbyopia, a condition in which the eye’s lens loses elasticity, affecting its ability to focus on near objects. Presbyopia typically occurs after age 40 and most patients use reading glasses in order to read or see objects close to them. There are no currently approved pharmacological therapies for presbyopia, but those in development plan to create a small pupil to better focus images on the retina via the “pinhole effect”. |
• | Potential to be the first oral therapy. Compared to intravitreal anti-VEGF injections, associated with systemic adverse events and ocular complications, twice a day oral administration of APX3330 could be a convenient alternative treatment for retinal disease, if approved. |
• | Upstream target implicated in two validated pathways. APX3330 is designed to lead to inhibition of two validated cell signaling pathways (angiogenesis and inflammation) known to cause various retinal diseases. Moreover, the APX3330 mechanism of action is distinct by working upstream of the current anti-VEGF therapies, thus Ocuphire believes it could complement anti-VEGF therapies and potentially reduce frequency of doctor visits. |
• | Promising tolerability profile. In 11 completed Phase 1 and Phase 2 clinical trials, APX3330 was well tolerated with no significant acute neurologic, cardiovascular, liver, or pulmonary events. |
• | Stable, cost-effective oral tablet. APX3330 is formulated as an oral tablet with stability suitable for eventual commercialization, and its active pharmaceutical ingredient is a small molecule with the advantages of standardized, scalable lower-cost manufacturing processes. |
• | DR, the leading cause of vision loss in adults aged 20–74 years, is the result of chronic elevations of glucose in the blood that lead to cell damage in the retina. |
• | DME, one of the most common complications of DR where there is vascular leakage in the macula, the part of the eye that is necessary for central and color vision. |
• | wAMD, a chronic eye disorder that causes visual distortions in the central part of one’s vision. It is usually caused by abnormal blood vessels that leak fluid or blood into the macula, the part of the eye that is necessary for central and color vision. |
• | Advance the clinical development of Nyxol and APX3330. Ocuphire is preparing to conduct registration studies of Nyxol and proof of concept studies of APX3330 with the objective of filing a U.S. NDA in early 2023 for Nyxol and advancing APX3330 towards an NDA in the future. |
• | Target Nyxol and APX3330 for large ophthalmic indications. Ocuphire believes Nyxol has therapeutic potential to improve vision performance in NVD, RM, and presbyopia. Ocuphire also believes AXP3330 has potential to improve the health of the retina in patients with diabetic retinopathy, diabetic macular edema and wAMD, while reducing the burden of intravitreal injections. |
• | Maintain and expand its intellectual property portfolio. Ocuphire owns all global patent rights to Nyxol with respect to its formulation, combinations, and use in multiple indications. Ocuphire also owns an exclusive worldwide sublicense for the Ref-1 Inhibitor program, including its lead product candidate APX3330, for all its ophthalmic and diabetic indications, and compositions and methods of use for Ref-1 pipeline candidates, including APX2009 and APX2014. Ocuphire continues to explore additional opportunities to expand and extend this intellectual property protection, both in the U.S. and in other jurisdictions. |
• | Maximize the global commercial value of Nyxol and APX3330. Ocuphire plans to seek commercial partners both in and outside of the United States. Alternatively, Ocuphire believes it could independently commercialize Nyxol and/or APX3330 in the United States with a targeted sales force. |
• | Evaluate in-licensing and acquisition opportunities. Ocuphire’s team is well qualified to identify and in-license or acquire clinical-stage ophthalmological assets and is evaluating opportunities to expand and diversify its pipeline. |
• | Non-proliferative DR, or NPDR. NPDR is an earlier, more typical stage of DR and can progress into more severe forms of DR over time if untreated and if exposure to elevated blood sugar levels persists. |
• | Proliferative DR, or PDR. PDR is a more advanced stage of DR than NPDR. It is characterized by retinal neovascularization and, if left untreated, leads to permanent damage and blindness. |
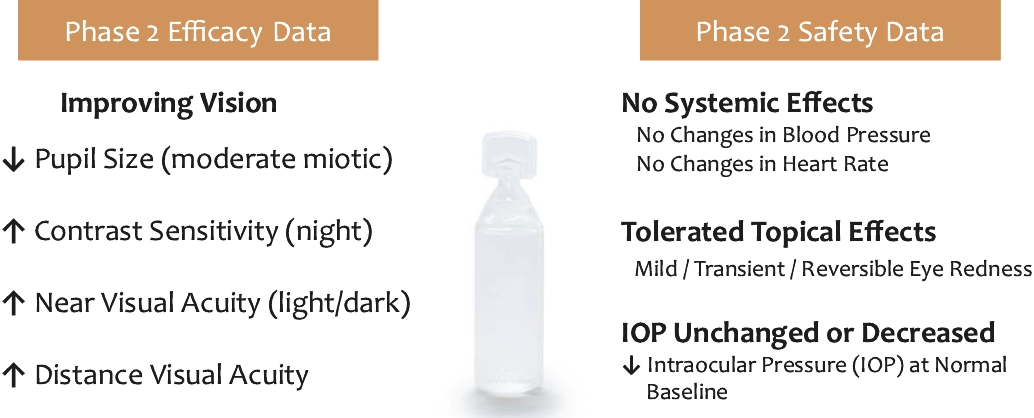
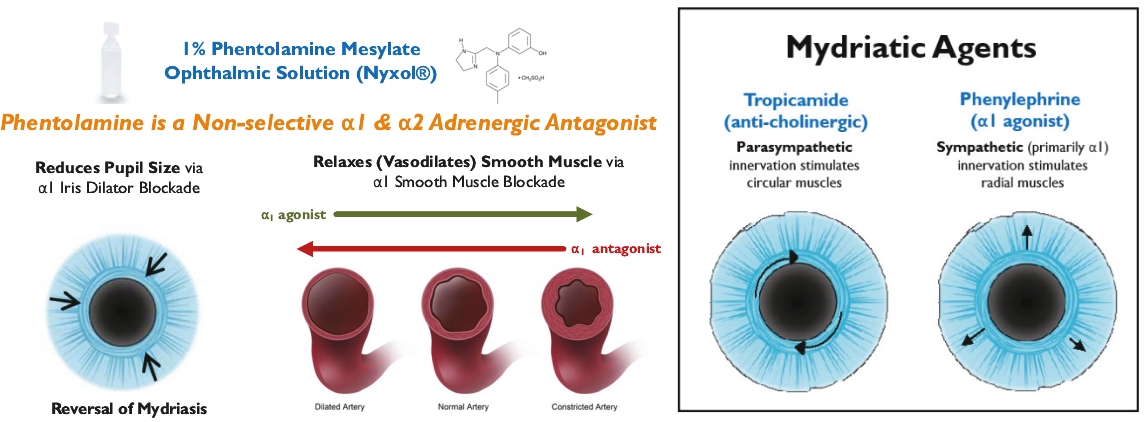
• | In a double-masked, randomized, single dose, 3-arm controlled, parallel design Phase 1 trial (OP-NYX-001, IND 67-288), 45 healthy volunteers were administered a single dose of 0.2% Nyxol with or without tetrahydrozoline or tetrahydrozoline alone. Both Nyxol-treated groups showed a statistically significant reduction in pupil diameter (PD) compared to tetrahydrozoline alone. |
• | In a 12-day, double-masked, randomized, placebo-controlled, single-dose, incomplete block, 3-period crossover, dose escalation Phase 1 trial in 16 healthy volunteers (OP-NYX-002, IND 67-288), there was a dose-related response in improvement in LCVA relative to placebo. |
• | In a 2-week, double-masked, randomized, placebo-controlled, single-dose, incomplete block 3-period crossover, dose escalation Phase 1/2 trial in 16 patients with NVD (OP-NYX-004, IND 73-987), Nyxol was well-tolerated with no severe adverse events (SAEs). |
• | In a 1-day, double-masked, randomized, placebo-controlled, single-dose Phase 2 trial in 24 patients with severe NVD (OP-NYX-SNV, IND 70-736), patients treated with Nyxol exhibited greater reductions in pupil diameter and greater improvements in low contrast visual acuity compared to those on placebo. |
• | In a 15-day, double-masked, randomized, placebo-controlled, multiple-dose, 3-arm (0, 0.5%, and 1% Nyxol) Phase 2 trial in 60 patients with severe NVD (OP-NYX-01a2, IND 70499), improvements in contrast sensitivity frequencies and VA, as well as reductions in intraocular pressure (IOP) and pupil diameter, were observed. |
• | In a 14-day, double-masked, randomized, placebo-controlled, multiple-dose, multi-center Phase 2b trial in 39 patients with elevated intraocular pressure (ORION-1, IND 070499), patients treated with 1% Nyxol showed statistically significant reduction in PD and improvement in near visual acuity relative to placebo, with evening bedtime daily dosing regimen. |
• | In a double-masked, randomized, placebo-controlled, crossover, single-dose, multi-center Phase 2b trial with 32 healthy patients (MIRA-1, IND 070499) to study reversal of pharmacologically induced mydriasis, healthy patients treated with 1% Nyxol had statistically significantly greater reductions in PD at multiple time points compared to placebo, and more patients in the study group returned to baseline PD at 2 hours compared to the placebo group. |
Trial Name (IND Number) | | | Patient / Indication | | | Phase | | | Trial Objectives | | | Doses | | | Number of Patients^ | | | Dosing | | | Key Endpoints |
NYX-001 (67-288) | | | Healthy Volunteers | | | 1 | | | Double-masked, randomized, single dose, 3-arm controlled, parallel trial to determine the efficacy and safety of phentolamine mesylate | | | 0.2% | | | Nyxol*=15, Visine=15, Visine + Nyxol*=15 Total = 45 | | | Single-dose | | | Safety and Efficacy (PD) |
NYX-002 (67-288) | | | Healthy Volunteers | | | 1 | | | Double-masked, randomized, placebo-controlled, single-dose, incomplete block, 3-period crossover, dose escalation trial evaluating the tolerability and efficacy of phentolamine mesylate | | | 0.2%, 0.4%, 0.8% | | | Nyxol*=16 Placebo=12 Total = 16 | | | Single-dose | | | Safety and Efficacy (PD, VA) |
OP-NYX-004 (73-987) | | | Night Vision Disturbances Patients | | | 1 / 2 | | | Double-masked, randomized, placebo-controlled, single-dose, incomplete block 3-period crossover, dose escalation trial to determine the efficacy and safety of phentolamine mesylate | | | 0.2%, 0.4%, 0.8% | | | Nyxol*=16 Placebo=12 Total = 16 | | | Single-dose | | | Safety and Efficacy |
OP-NYX-SNV (70-736) | | | Severe Night Vision Disturbances Patients | | | 2 | | | Double-masked, randomized, placebo-controlled, single-dose trial to assess the efficacy and safety of phentolamine mesylate ophthalmic solution | | | 1.0% | | | Nyxol*=16, Placebo=8 Total = 24 | | | Single-dose | | | Safety and Efficacy (PD, LCVA, CS, WA) |
OP-NYX-01a2 (70-499) | | | Severe Night Vision Disturbances Patients | | | 2 | | | Double-masked, randomized, placebo-controlled, single-dose, 3-arm trial to assess the efficacy and safety of Nyxol | | | 0.5%, 1.0% | | | Nyxol=40 Placebo=20 Total = 60 | | | Multiple doses (15-28 days) | | | Safety and Efficacy (PD, LCVA, CS) |
OPI-NYXG-201 (ORION-1) (70-499) | | | Glaucoma and Ocular Hypertension, Elderly Patients | | | 2b | | | Double-masked, randomized, placebo-controlled, multiple-dose, multi-center trial to assess the efficacy and safety of Nyxol | | | 1.0% | | | Nyxol=19 Placebo=20 Total = 39 | | | Multiple doses (14 days) | | | Safety and Efficacy (IOP, PD, near VA, VA) |
OPI- NYXRM-201 (MIRA-1) (70-499) | | | Healthy Patients/ Reversal of Mydriasis | | | 2b | | | Double-masked, randomized, placebo-controlled, crossover, single-dose, multi-center trial to assess the efficacy and safety of Nyxol in reducing pharmacologically induced mydriasis | | | 1.0% | | | Nyxol=31 Placebo=32 Total = 32 | | | Single-dose | | | Safety and Efficacy (PD, Accommodation, VA) |
Study | | | Group | | | Mesopic Conditions | ||||||||||||
| | Pre-Treatment (Baseline) Pupil Diameter | | | Post- Treatment Pupil Diameter | | | Change (%) | | | p-value compared to baseline | | | p-value compared to placebo | |||||
NYX-SNV | | | Placebo (N = 16) | | | 6.6mm | | | 6.4mm | | | -0.2mm (-3%) | | | p = 0.08 | | | p < 0.0001 |
| | | 1% Nyxol (N = 32) | | | 6.5mm | | | 5.2mm | | | -1.3mm (-20%) | | | p < 0.0001 | | | ||
NYX-01a2 | | | Placebo (N = 38) | | | 6.25mm | | | 6.31mm | | | 0.07mm (+1%) | | | p = 0.6 | | | p < 0.0001 |
| | | 1% Nyxol (N = 40) | | | 6.17mm | | | 5.31mm | | | -0.86mm (-14%) | | | p < 0.0001 | | | ||
NYXG-201 | | | Placebo (N = 20) | | | 4.57mm | | | 4.52mm | | | -0.05mm (-1%) | | | p = 0.6178 | | | p < 0.0001 |
| | | 1% Nyxol (N = 19) | | | 4.69mm | | | 3.70mm | | | -1.00mm (-21%) | | | p < 0.0001 | | | ||
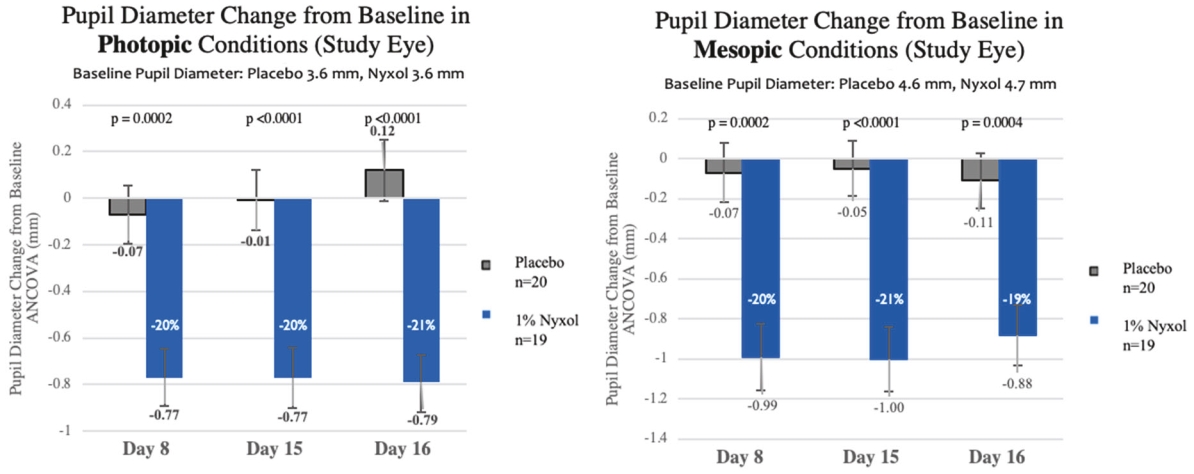
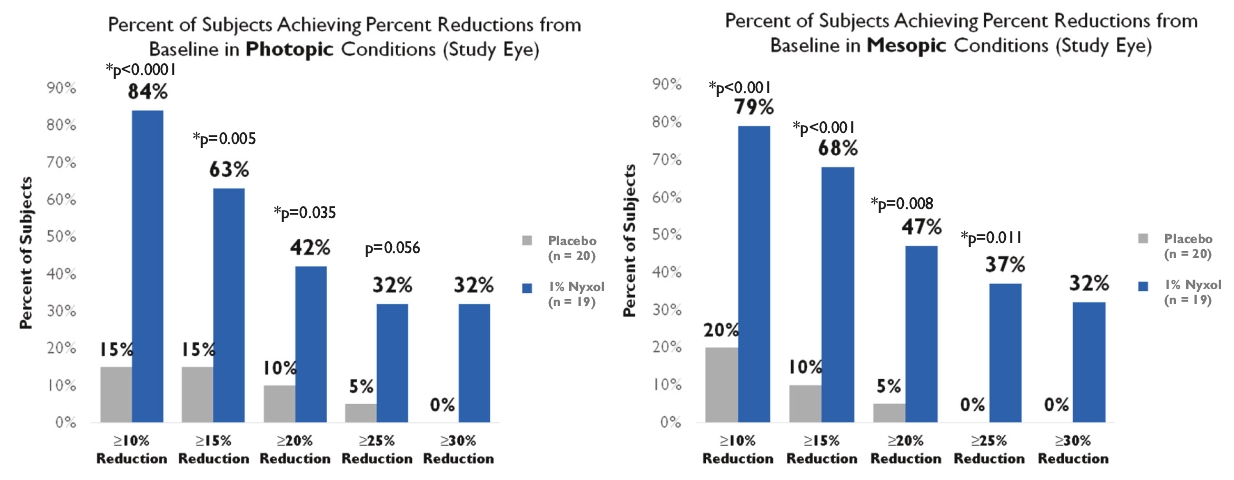
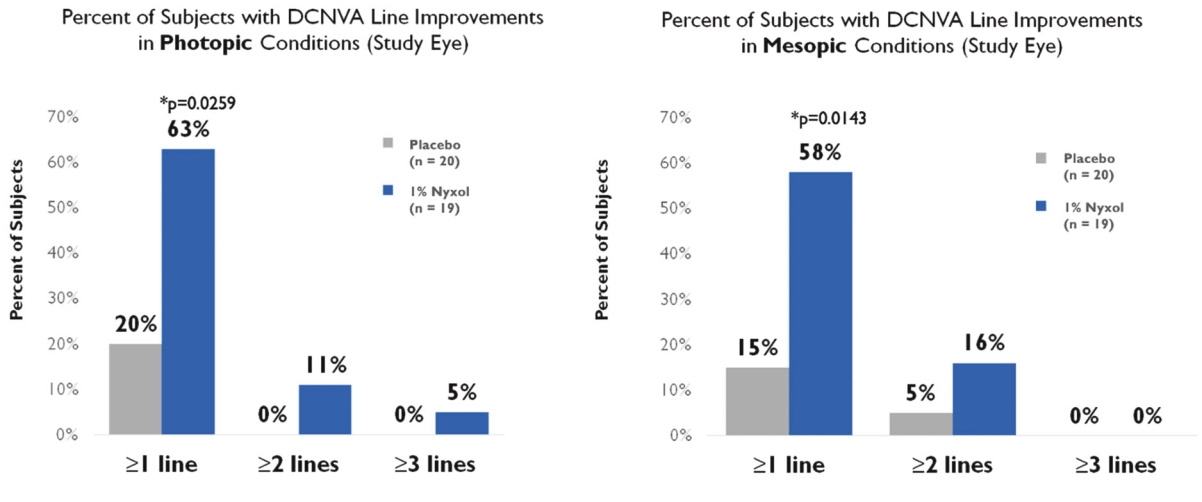
System Organ Class Preferred Term | | | Nyxol (n=19) n (%) | | | Placebo (n=20) n (%) |
Total number of TEAEs, n[1] | | | 16 | | | 2 |
Eye disorders | | | 3 (15.8) | | | 1 (5.0) |
Conjunctival hyperemia | | | 3 (15.8) | | | 1 (5.0) |
Eye pruritus | | | 1 (5.3) | | | 0 |
Vision blurred | | | 0 | | | 0 |
Conjunctival hemorrhage | | | 0 | | | 0 |
Corneal deposits | | | 0 | | | 0 |
Erythema of eyelid | | | 0 | | | 0 |
Eye irritation | | | 0 | | | 0 |
Eyelid edema | | | 0 | | | 0 |
System Organ Class Preferred Term | | | Nyxol (n=19) n (%) | | | Placebo (n=20) n (%) |
Lacrimation increased | | | 0 | | | 0 |
Eye pain | | | 0 | | | 0 |
Visual acuity reduced | | | 0 | | | 0 |
Conjunctival edema | | | 0 | | | 0 |
Foreign body sensation in eyes | | | 0 | | | 0 |
Punctate keratitis | | | 0 | | | 0 |
General disorders and administration site conditions | | | 3 (15.8) | | | 0 |
Instillation site burn | | | 2 (10.5) | | | 0 |
Instillation site pain | | | 1 (5.3) | | | 0 |
Infections and infestations | | | 1 (5.3) | | | 0 |
Prostate infection | | | 1 (5.3) | | | 0 |
Upper respiratory tract infection | | | 1 (5.3) | | | 0 |
Nervous system disorders | | | 0 | | | 0 |
Headache | | | 0 | | | 0 |
Skin and subcutaneous tissue disorders | | | 0 | | | 0 |
Injury, poisoning and procedural complication | | | 0 | | | 0 |
Respiratory, thoracic, and mediastinal disorders | | | 0 | | | 0 |
Cardiac disorders | | | 0 | | | 0 |
Vascular disorders | | | 0 | | | 0 |
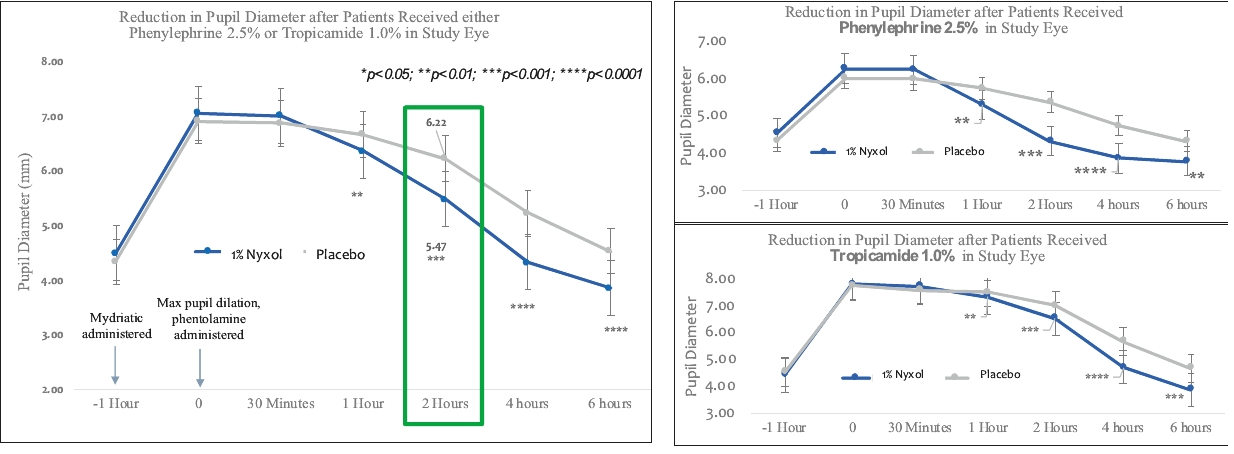
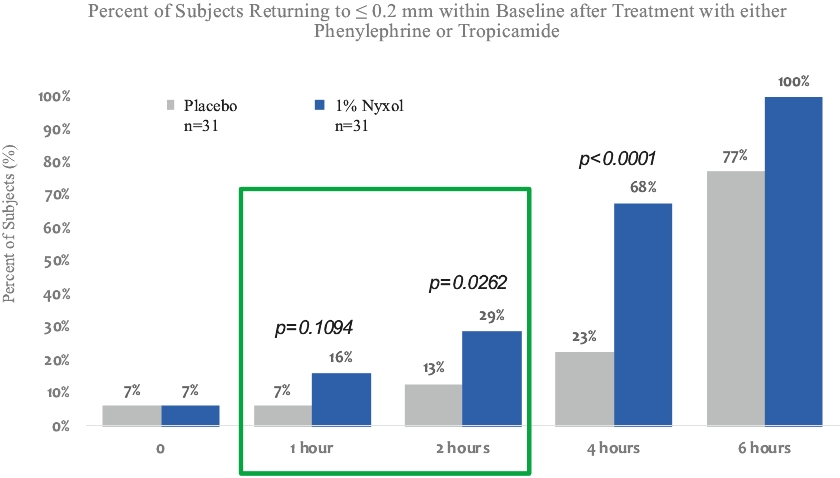
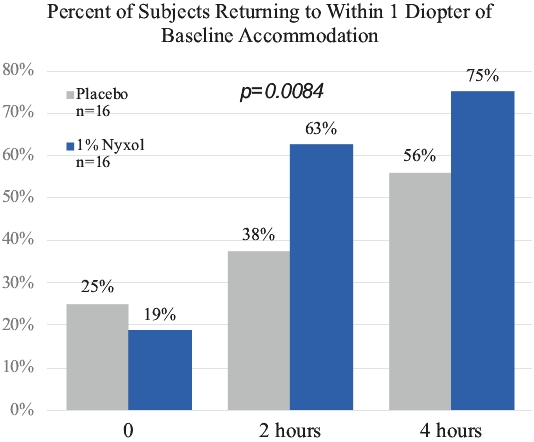
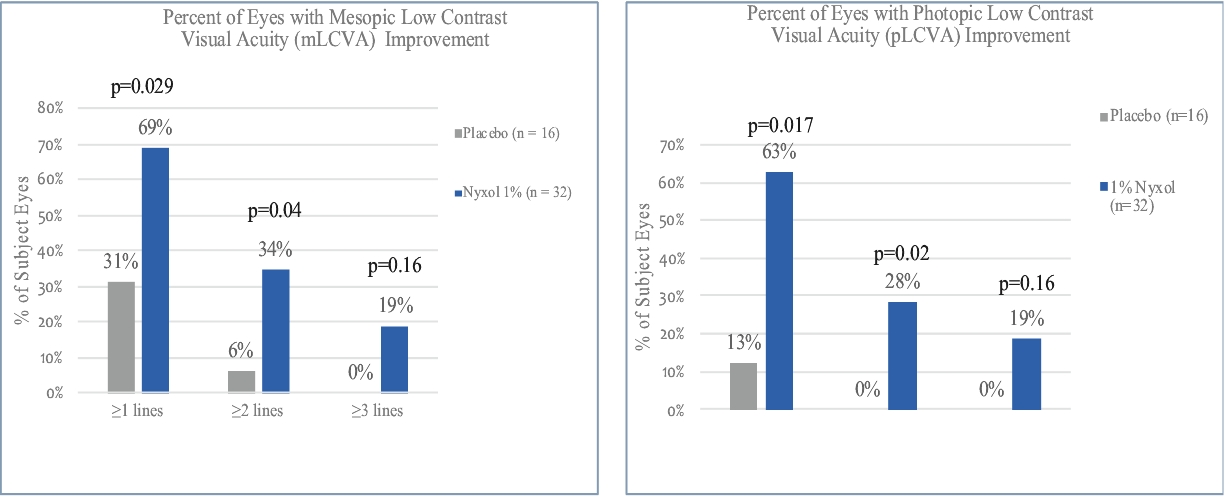
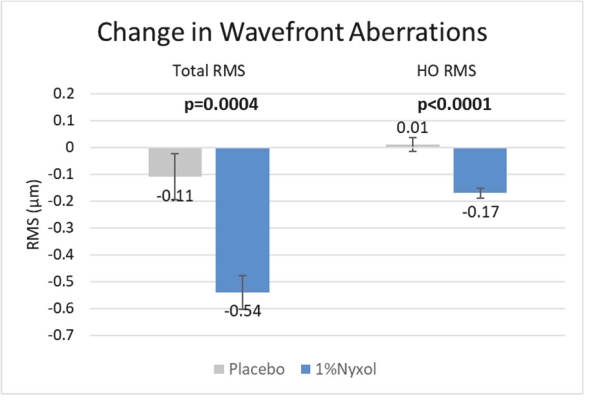
Variable | | | Placebo (N = 40) | | | 0.5% Nyxol (N = 40) | | | 1% Nyxol (N = 40) |
Pre-Treatment Day 1 IOP (mmHg ± STDEV) | | | 16.1 ± 2.3 | | | 16.7 ± 2.7 | | | 16.6 ± 2.5 |
Post-Treatment Day 1 IOP (mmHg ± STDEV) | | | 16.2 ± 3.2 | | | 15.4 ± 3.6 | | | 14.2 ± 2.9 |
Change from Pretreatment Day 1 IOP (mmHg ± STDEV) | | | 0.1 ± 2.7 | | | -1.3 ± 3.2 | | | -2.4 ± 2.2 |
Change in Baseline Significance^ | | | p = 0.9192 | | | p = 0.0043 | | | p < 0.0001 |
Change compared to Placebo Significance^ | | | N/A | | | p = 0.0148 | | | p < 0.0001 |
^ | P-values were generated using the Wilcoxon Signed Rank Test. |
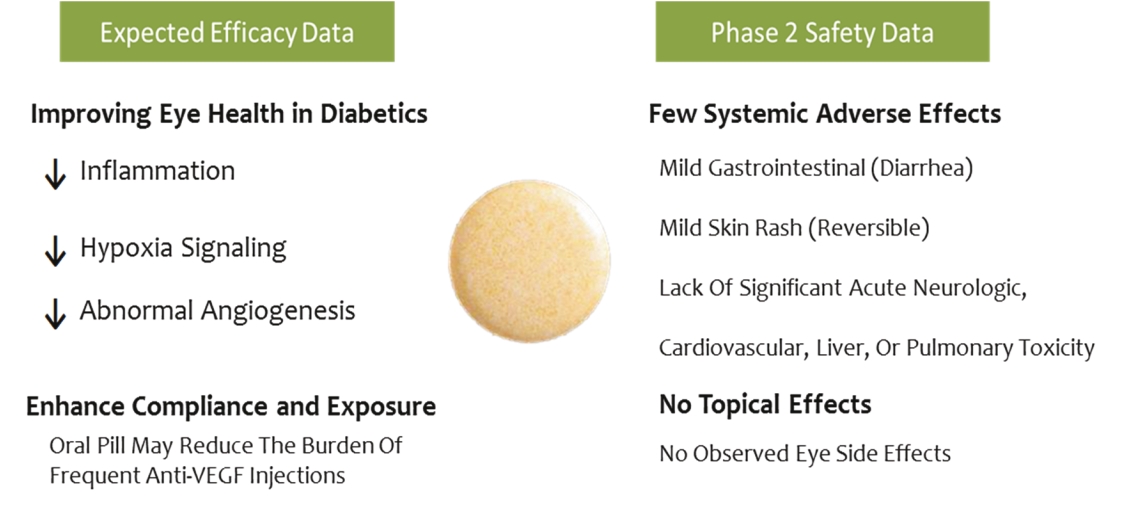
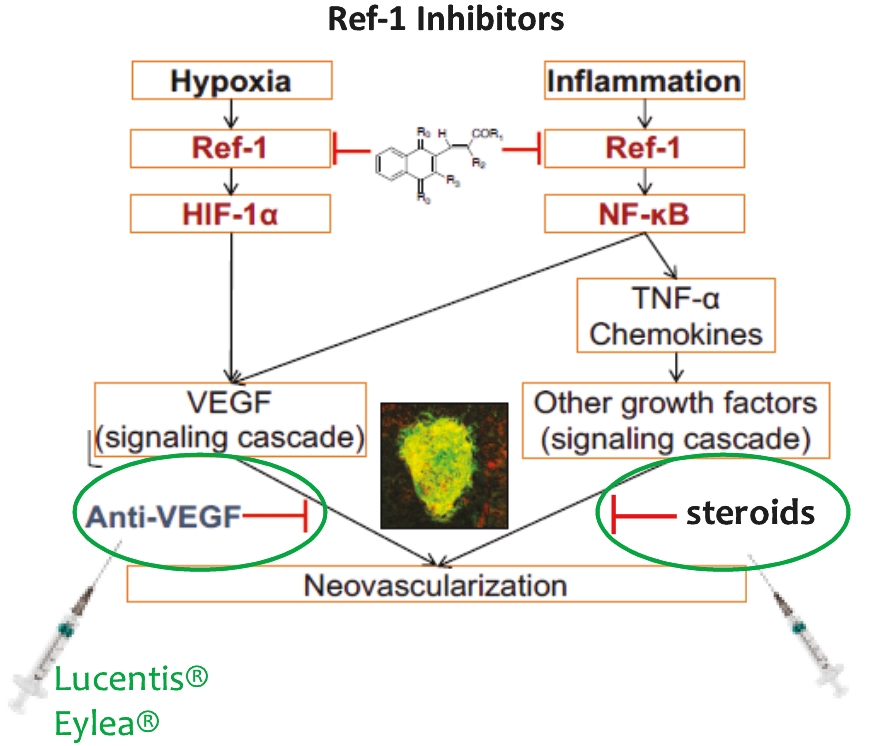
• | APX_CLN_0001: A Phase 1, randomized, single-dose placebo-controlled trial of APX3330 to investigate the safety and pharmacokinetics during oral dosing of APX3330 to healthy adult males. A total of 18 patients were treated with single oral doses of APX3330 (10 mg, 30 mg, 60 mg, 120 mg, 180 mg or 240 mg) or the placebo in a blind manner. |
• | APX_CLN_0002: An 8-day, randomized Phase 1 repeat-dose placebo-controlled trial to investigate the safety and pharmacokinetics of orally dosed APX3330 in healthy adult male patients. A total of 18 patients were treated with oral dosing of APX3330 (120 mg or 240 mg) or the placebo in a blind manner once or twice a day for 8 successive days. |
• | APX_CLN_0003: A 7-day Phase 1 repeat-dose trial (120 mg) in 6 healthy patients to determine the effects of food on orally administered APX3330. |
• | APX_CLN_0004 A single-dose trial (120 mg) in 6 healthy patients to determine the effect of meals on the pharmacokinetics of APX3330. |
• | APX_CLN_0005 A 12-week dose-escalation Phase 2 trial (20 mg, 60 mg, 120 mg, 240 mg) in 40 chronic hepatitis B patients. Patients received oral administration of one tablet per dose (2 tablets in the case of the administration of 240 mg) twice a day, after breakfast and after dinner. |
• | APX_CLN_0006 A 12-week dose-escalation Phase 2 trial (20 mg, 60 mg, 120 mg, 240 mg) in 51 chronic hepatitis C patients. The objective of the trial was to investigate the safety, efficacy and utility of APX3330 in treating patients with chronic hepatitis C. |
• | APX_CLN_0007 A 12-week double-masked, randomized placebo-controlled Phase 2 trial (0 mg, 120 mg, 240 mg) in chronic hepatitis C patients that had failed previous interferon treatment. Safety was evaluated in 196 completed patients. The mean treatment period in each group was 82 days in the placebo group, 79 days in the 120 mg group and 78 days in the 240 mg group. The primary endpoints of this trial were measurement of the rate of change in the glutamic pyruvate transaminase (GPT) level, degree of improvement in liver function and assessment of general performance status. |
• | APX_CLN_0008 A 3-step, Phase 1 single-dose, single-blind trial (300 mg, 420 mg, 600 mg) in 27 healthy patients to investigate the safety and pharmacokinetics of higher doses. |
• | APX_CLN_0009 A 2-week repeated-dose Phase 2 trial (120 mg) in 30 patients with acute severe hepatitis, including patients with advanced liver cirrhosis. Efficacy endpoints included objective measures of liver function and subjective improvement of patient functional status. Safety measures included the assessment of the general tolerability of the drug (i.e., changes in vital signs) and changes in clinical laboratory values. |
• | APX_CLN_00010 A 4-week repeated-dose Phase 2 trial (120 mg) in 30 patients with alcoholic hepatitis, including patients with liver cirrhosis. Efficacy endpoints included objective measures of liver function and subjective improvement of patient functional status. Safety measures included the assessment of the general tolerability of the product candidate (i.e., changes in vital signs) and changes in clinical laboratory values. |
• | APX_CLN_0011 was a multi-center, open-label, dose-escalation Phase 1 oncology trial in patients with advanced solid tumors. Patients received daily oral doses of APX3330 each day of repeated 21-day cycles until disease progression or trial withdrawal. Nineteen patients received APX3330 in escalating doses from 240 mg/d dose to 720 mg/d in increments of 120mg/d. The top dose tested (720 mg/d) produced a self-limiting, diffuse macular rash and was confirmed as the dose-limiting toxicity. The dose of 600 mg/d was then confirmed as a dose tolerable for chronic administration and for further clinical development as a modulator of Ref-1 activity in inflammatory diseases. Biopsy analyses of patients participating in the trial confirmed that APX3330 directly targets the Ref-1 protein and that the targeting produces subsequent regulation of transcription factors such as NF-κB and HIF-1α, regulators of VEGF and other inflammatory molecules. This mechanism of action provides significant rationale for testing APX3330 in diseases in which inflammation and neo-vascular development play a critical pathogenic role. |
Trial Number / Name | | | Patient / Indication | | | Phase | | | Trial Objectives | | | Doses | | | Number of Patients | | | Dosing | | | Key Endpoints |
APX_CLN_0001 | | | Healthy Volunteers | | | 1 | | | Single-dose placebo-controlled trial of APX3330 to investigate safety and pharmacokinetics | | | 10 mg 30 mg 60 mg 120 mg 180 mg 240 mg | | | APX3330 = 9 Placebo = 9 | | | Single dose | | | Plasma Concentration of total quinone forms, safety |
APX_CLN_0002 | | | Healthy Volunteers | | | 1 | | | Repeat-dose placebo-controlled trial to investigate safety and pharmacokinetics | | | 120 mg QD 120 mg BID | | | APX3330 = 9 Placebo = 9 | | | 8 days | | | Plasma Concentration of APX3330, safety |
APX_CLN_0003 | | | Healthy Volunteers | | | 1 | | | Repeat-dose trial to determine effects of food on pharmacokinetics | | | 240 mg | | | APX3330 = 6 | | | 1 week | | | Plasma Concentration of APX3330, safety |
APX_CLN_0004 | | | Healthy Volunteers | | | 1 | | | Single-dose trial to determine the effects of meals on pharmacokinetics | | | 120 mg | | | APX3330 = 6 | | | Single dose | | | Plasma Concentration of APX3330, Safety |
APX_CLN_0005 | | | Chronic Hepatitis B Patients | | | 2 | | | Dose-escalation trial to investigate safety, efficacy and tolerability | | | 20 mg 60 mg 120 mg 240 mg | | | APX3330 = 40 | | | 12 weeks | | | Safety |
APX_CLN_0006 | | | Chronic Hepatitis C Patients | | | 2 | | | Dose-escalation trial to investigate safety, efficacy and tolerability | | | 20 mg 60 mg 120 mg 240 mg | | | APX3330 = 51 | | | 12 weeks | | | Safety |
Trial Number / Name | | | Patient / Indication | | | Phase | | | Trial Objectives | | | Doses | | | Number of Patients | | | Dosing | | | Key Endpoints |
APX_CLN_0007 | | | Chronic Hepatitis C Patients | | | 2 | | | Double-masked, placebo-controlled trial to investigate safety, efficacy and tolerability | | | 120 mg 240 mg | | | APX3330 = 128 Placebo = 68 | | | Placebo = 82 days APX3330 120 mg = 79 days 240 mg = 78 days | | | Rate of change in GPT level, improvement in liver function, general performance |
APX_CLN_0008 | | | Healthy Patients | | | 1 | | | Single-blind, single-dose, 3-step trial to investigate safety and pharmacokinetics of higher doses | | | 300 mg 420 mg 600 mg | | | APX3330 = 27 | | | Single dose | | | Plasma Concentration of APX3330, safety |
APX_CLN_0009 | | | Advanced Liver Cirrhosis Patients | | | 2 | | | Repeated-dose trial to investigate safety, efficacy and tolerability | | | 120 mg | | | APX3330 = 30 | | | 2 weeks | | | Liver function, patient functional status, tolerability |
APX_CLN_0010 | | | Advanced Liver Cirrhosis Patients | | | 2 | | | Repeated-dose trial to investigate safety, efficacy and tolerability | | | 120 mg | | | APX3330 = 30 | | | 4 weeks | | | Liver function, patient functional status, tolerability |
APX_CLN_0011 | | | Advanced Solid Tumor Patients | | | 1 | | | Multicenter, open-label, dose-escalation to investigate safety, efficacy, pharmacokinetics, and recommended Phase 2 dose | | | 240 mg 360 mg 480 mg 600 mg 720 mg | | | APX3330 = 19 | | | 21-day cycles until disease progression or study withdrawal | | | Tumor response, safety, PK, target engagement |
System Organ Class Preferred Term | | | APX3330 20-240 mg (N=279) | | | Placebo (N=68) | ||||||
| | n (%) | | | # events | | | n (%) | | | # events | ||
Adverse Events | | | 40 (14.3) | | | 52 | | | 11 (16.2) | | | 15 |
Blood and lymphatic system disorders | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Anemia | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Cardiac disorders | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Palpitations | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Gastrointestinal disorders | | | 12 (4.3) | | | 14 | | | 2 (2.9) | | | 2 |
Abdominal discomfort | | | 1 (0.4) | | | 1 | | | 1 (1.5) | | | 1 |
Abdominal pain | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Abdominal pain lower | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Cheilitis | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Diarrhea | | | 3 (1.1) | | | 3 | | | 0 | | | 0 |
Feces soft | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Gastric ulcer | | | 2 (0.7) | | | 2 | | | 0 | | | 0 |
Hypo aesthesia oral | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Mouth swelling | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Stomatitis | | | 0 | | | 0 | | | 1 (1.5) | | | 1 |
Tongue dry | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Vomiting | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
General disorders and administration site conditions | | | 6 (2.2) | | | 6 | | | 3 (4.4) | | | 3 |
Chest discomfort | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Feeling abnormal | | | 0 | | | 0 | | | 1 (1.5) | | | 1 |
Malaise | | | 3 (1.1) | | | 3 | | | 1 (1.5) | | | 1 |
Peripheral edema | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Peripheral swelling | | | 0 | | | 0 | | | 1 (1.5) | | | 1 |
Pyrexia | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Infections and infestations | | | 3 (1.1) | | | 3 | | | 0 | | | 0 |
Nasopharyngitis | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Upper respiratory tract infections | | | 2 (0.7) | | | 2 | | | 0 | | | 0 |
Investigations | | | 2 (0.7) | | | 2 | | | 0 | | | 0 |
Blood urea increased | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Urobilinogen urine increased | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Musculoskeletal and connective tissue disorders | | | 0 | | | 0 | | | 2 (2.9) | | | 3 |
Limb discomfort | | | 0 | | | 0 | | | 1 (1.5) | | | 1 |
Musculoskeletal pain | | | 0 | | | 0 | | | 1 (1.5) | | | 1 |
Pain in extremity | | | 0 | | | 0 | | | 1 (1.5) | | | 1 |
Nervous system disorders | | | 4 (1.4) | | | 6 | | | 4 (5.9) | | | 5 |
Ageusia | | | 0 | | | 0 | | | 1 (1.5) | | | 1 |
Burning sensation | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Dizziness | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Headache | | | 2 (0.7) | | | 2 | | | 1 (1.5) | | | 1 |
Hypoaesthesia | | | 1 (0.4) | | | 1 | | | 1 (1.5) | | | 1 |
Hypoglycemic coma | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Parosmia | | | 0 | | | 0 | | | 1 (1.5) | | | 1 |
Subarachnoid hemorrhage | | | 0 | | | 0 | | | 1 (1.5) | | | 1 |
Eye disorders | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Ocular discomfort | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Psychiatric disorders | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Insomnia | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Renal and urinary disorders | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Hematuria | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Respiratory, thoracic and mediastinal disorders | | | 2 (0.7) | | | 2 | | | 1 (1.5) | | | 1 |
Acute respiratory distress syndrome | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Upper respiratory tract inflammation | | | 1 (0.4) | | | 1 | | | 1 (1.5) | | | 1 |
System Organ Class Preferred Term | | | APX3330 20-240 mg (N=279) | | | Placebo (N=68) | ||||||
| | n (%) | | | # events | | | n (%) | | | # events | ||
Skin and subcutaneous tissue disorders | | | 12 (4.3) | | | 14 | | | 1 (1.5) | | | 1 |
Alopecia | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Drug eruption | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Dry skin | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Eczema | | | 2 (0.7) | | | 2 | | | 0 | | | 0 |
Papule | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
Pruritus | | | 5 (1.8)) | | | 5 | | | 1 (1.5) | | | 1 |
Rash | | | 2 (0.7) | | | 2 | | | 0 | | | 0 |
Urticaria | | | 1 (0.4) | | | 1 | | | 0 | | | 0 |
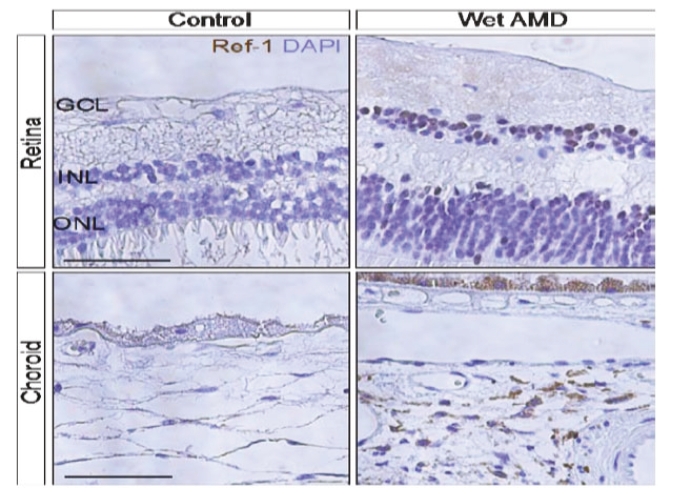
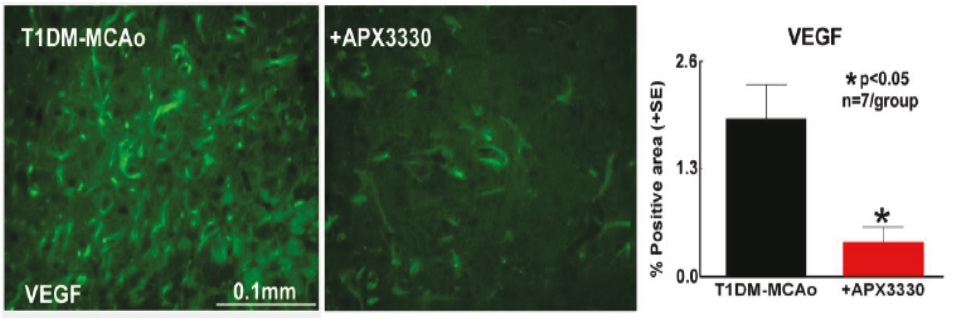
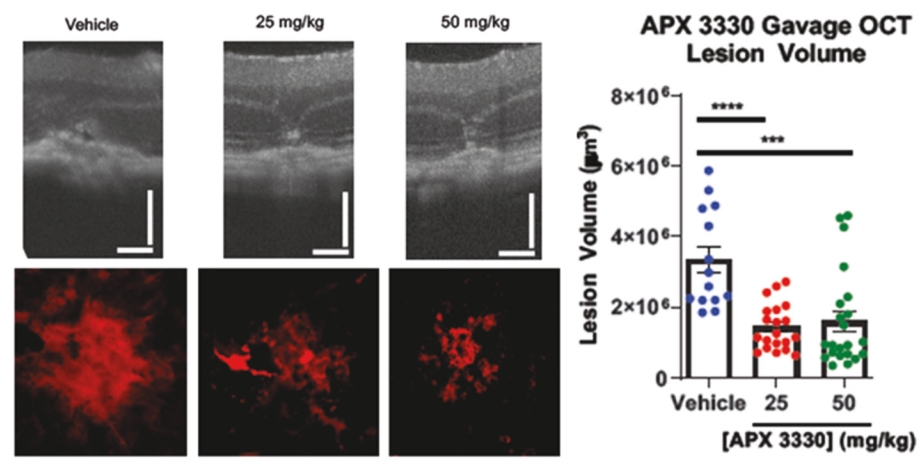
• | Presbysol® (AGN-190584), with 1.25% pilocarpine, developed by Allergan plc. |
• | Presbidrops® (CSF-1), with low dose pilocarpine and a secondary agent (lubricant), developed by Orasis Pharmaceuticals Ltd. |
• | Liquid Vision®, with aceclidine (another miotic agent), developed by Presbyopia Therapies, LLC. |
• | MicroLine®, which is a microdose formulation of pilocarpine, developed by Eyenovia, Inc. |
• | KT-101, which uses pilocarpine in the AcuStream delivery system, developed by Kedalion Therapeutics, Inc. |
• | UNR844, which uses a mechanism that involves softening the lens to increase near visual acuity, developed by Novartis AG (originally Encore Vision, Inc.). |
1. | Lucentis® (ranibizumab) and Avastin® (bevacizumab), which are anti-VEGF monoclonal antibody intravitreal injections, developed by Genentech, Inc. |
2. | EYLEA® (aflibercept), a VEGF inhibitor intravitreal injection, developed by Regeneron Pharmaceuticals. |
3. | Beovu® Brolucizumab, an anti-VEGF monoclonal antibody intravitreal injection, developed by Novartis AG. |
4. | MACUGEN® (pegaptanib sodium injection), a selective inhibitor of VEGF-165, developed by Bausch + Lomb. |
5. | Ozurdex® (dexamethasone), a corticosteroid IVT implant, developed by Allergan plc. |
6. | Iluvien (fluocinolone acetonide), a corticosteroid IVT implant, developed by Alimera Sciences, Inc. |
• | Abicipar pegol, an anti-VEGF intravitreal injection with a long duration of action, developed by Allergan plc and Molecular Partners. |
• | Farcimab, a bispecific antibody intravitreal injection that suppresses both VEGF and Angiopoietin-2, developed by Genentech, Inc. and Roche AG. |
• | KSI-301, an anti-VEGF antibody intravitreal injection coupled with a biopolymer that is intended to increase the time between injections, developed by Kodiak Sciences. |
• | OPT-302, an intravitreal injection which binds to multiple types of VEGF receptors that could be used with other anti-VEGF agents, developed by Opthea Limited. |
• | ALG-1001, an integrin peptide therapy intravitreal injection that is being evaluated as a sequential or in-combination therapy with bevacizumab in patients with DME, developed by Allegro Ophthalmics, LLC. |
• | completion of preclinical laboratory tests, animal studies and formulation studies in compliance, as applicable, with the Animal Welfare Act and FDA’s good laboratory practice, or GLP, regulations; |
• | submission to the FDA of an IND, which must take effect before human clinical trials may begin; |
• | approval by an independent institutional review board, or IRB, representing each clinical site before each clinical trial may be initiated; |
• | performance of adequate and well-controlled human clinical trials in accordance with good clinical practices, or GCP, to establish the safety and efficacy of the proposed drug product for each indication; |
• | preparation and submission to the FDA of an NDA; |
• | review of the product by an FDA advisory committee, where appropriate or if applicable; |
• | satisfactory completion of one or more FDA inspections of the manufacturing facility or facilities at which the product, or components thereof, are produced to assess compliance with current Good Manufacturing Practices, or cGMP, requirements and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity; |
• | satisfactory completion of FDA audits of clinical trial sites to assure compliance with GCPs and the integrity of the clinical data; |
• | payment of user fees and securing FDA approval of the NDA; and |
• | compliance with any post-approval requirements, including Risk Evaluation and Mitigation Strategies, or REMS, and post-approval studies required by the FDA. |
• | Phase 1. The drug is initially introduced into healthy human patients or, in certain indications such as cancer, patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness and to determine optimal dosage. |
• | Phase 2. The drug is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
• | Phase 3. The drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product and to provide adequate information for the labeling of the product. |
• | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
• | fines, warning letters or holds on post-approval clinical trials; |
• | refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product license approvals; |
• | product seizure or detention, or refusal to permit the import or export of products; or |
• | injunctions or the imposition of civil or criminal penalties. |
• | the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid; |
• | the federal civil and criminal false claims laws, including the civil False Claims Act, and civil monetary penalties laws, which prohibit individuals or entities from, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
• | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created additional federal criminal laws that prohibit, among other things, knowingly and willingly executing, or attempting to execute, a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
• | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, which also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
• | the federal transparency requirements known as the federal Physician Payments Sunshine Act, under the Patient Protection and Affordable Care Act, as amended by the Health Care Education Reconciliation Act, or the Affordable Care Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies to report specially to the Centers for Medicare & Medicaid Services, or CMS, within the U.S. Department of Health and Human Services, information related to payments and other transfers of value to clinicians and teaching hospitals and clinician ownership and investment interests; and |
• | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to healthcare items or services that are reimbursed by non-governmental third-party payors, including private insurers. |
• | a special, nondeductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs, although this fee would not apply to sales of certain products approved exclusively for orphan indications; |
• | expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to certain individuals with income at or below 133% of the federal poverty level, thereby potentially increasing a manufacturer’s Medicaid rebate liability; |
• | expanded manufacturers’ rebate liability under the Medicaid Drug Rebate Program by increasing the minimum rebate for both branded and generic drugs and revising the definition of “average manufacturer price,” or AMP, for calculating and reporting Medicaid drug rebates on outpatient prescription drug prices and extending rebate liability to prescriptions for individuals enrolled in Medicare Advantage plans; addressed a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected; |
• | expanded the types of entities eligible for the 340B drug discount program; |
• | established the Medicare Part D coverage gap discount program by requiring manufacturers to provide a 50% point-of-sale-discount off the negotiated price of applicable brand drugs to eligible beneficiaries during their coverage gap period as a condition for the manufacturers’ outpatient drugs to be covered under Medicare Part D; |
• | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; |
• | the Independent Payment Advisory Board, or IPAB, which has authority to recommend certain changes to the Medicare program to reduce expenditures by the program that could result in reduced payments for prescription drugs. However, the IPAB implementation has been not been clearly defined. The ACA provided that under certain circumstances, IPAB recommendations will become law unless Congress enacts legislation that will achieve the same or greater Medicare cost savings; and |
• | established the Center for Medicare and Medicaid Innovation within CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending. Funding has been allocated to support the mission of the Center for Medicare and Medicaid Innovation from 2011 to 2019. |
• | RX-3117 is a novel, investigational oral, small molecule nucleoside compound. Once intracellularly activated (phosphorylated) by the enzyme UCK2, it is incorporated into the DNA or RNA of cells and inhibits both DNA and RNA synthesis, which induces apoptotic cell death of tumor cells. RX-3117 has been the subject of a Phase 2a clinical trial in combination with Celgene’s ABRAXANE® (paclitaxel protein-bound particles for injectable suspension) as a first-line treatment in patients newly diagnosed with metastatic pancreatic cancer. The trial reached its target enrollment in February 2019. As of July 24, 2019, an overall response rate of 23% had been observed in 40 patients that had at least one scan on treatment. Preliminary and unaudited data indicates that the median progression free survival for patients in the study is approximately 5.4 months. Complete data from the trial is expected to be available in 2020. Rexahn does not plan to conduct or sponsor any additional trials with RX-3117. |
• | RX-5902 is a potential small molecule modulator of the Wnt/beta-catenin pathway which plays a key role in cancer cell proliferation and tumor growth. In August 2018, Rexahn entered into the Collaboration Agreement with Merck to evaluate the combination of RX-5902 and Merck’s anti-PD-1 therapy, KEYTRUDA® (pembrolizumab) in a Phase 2 trial in patients with metastatic TNBC. On April 7, 2020, Rexahn notified Merck that it was terminating the Collaboration Agreement, effective immediately, in connection with Rexahn’s determination to discontinue development of RX-5902 for the treatment of TNBC. Rexahn does not plan to conduct or sponsor any additional trials with RX-5902. |
• | RX-0301 is a potential potent inhibitor of the synthesis of the protein kinase Akt-1, which Rexahn believes plays a critical role in cancer cell proliferation, survival, angiogenesis, metastasis, and drug resistance. RX-0301 is currently in preclinical development by HaiChang as a nano-liposomal formulation of RX-0201 (Archexin®) using HaiChang’s proprietary QTsome™ technology. On February 8, 2020, Rexahn entered into the HaiChang Agreement, pursuant to which Rexahn granted HaiChang an exclusive (even as to Rexahn), royalty-bearing, sublicensable worldwide license to research, develop and commercialize RX-0201 and RX-0301. The HaiChang Agreement supersedes a prior agreement with HaiChang to develop RX-0301 under which HaiChang was to conduct certain preclinical and clinical activities through completion of a Phase 2a proof-of-concept clinical trial in HCC. |
• | CROs and investigative sites in connection with clinical studies; |
• | vendors in connection with product manufacturing, development, and distribution of clinical supplies; and |
• | vendors in connection with preclinical development activities. |
| | | For the Three Months Ended June 30, | | | For the Six Months Ended June 30, | |||||||
| | | 2020 | | | 2019 | | | 2020 | | | 2019 | |
Clinical Candidates: | | | | | | | | | ||||
RX-3117 | | | $86,000 | | | $1,058,000 | | | $412,900 | | | $2,136,400 |
RX-5902 | | | 7,500 | | | 187,800 | | | 11,700 | | | 530,200 |
RX-0201 | | | | | 55,300 | | | 1,800 | | | 171,100 | |
| | | | | | | | | |||||
Preclinical, Personnel and Overhead | | | 138,107 | | | 347,301 | | | 261,997 | | | 1,052,931 |
| | | | | | | | | |||||
Total Research and Development Expenses | | | $231,607 | | | $1,648,401 | | | $688,397 | | | $3,890,631 |
| | | For the Year Ended December 31, | ||||
| | | 2019 | | | 2018 | |
Clinical Candidates: | | | | | ||
RX-3117 | | | $3,088,900 | | | $6,126,200 |
RX-5902 | | | 887,200 | | | 3,104,400 |
RX-0201 | | | 175,600 | | | 651,200 |
Preclinical, Personnel and Overhead | | | 1,325,076 | | | 3,227,258 |
Total Research and Development Expenses | | | $5,476,776 | | | $13,109,058 |
| | | For the Year Ended December 31, | ||||
| | | 2019 | | | 2018 | |
Net Cash Used in Operating Activities | | | $(10,277,133) | | | $(18,838,638) |
Net Cash Provided By Investing Activities | | | 3,098,551 | | | 11,910,996 |
Net Cash Provided by Financing Activities | | | 7,653,828 | | | 6,772,789 |
Net Increase (Decrease) in Cash and Cash Equivalents | | | $475,246 | | | $(154,853) |
• | continues clinical trials for Nyxol, APX3330 and for any other product candidate in its future pipeline; |
• | continues preclinical studies for Nyxol, APX3330 and for any other product candidate in its future pipeline; |
• | develops additional product candidates that it identifies, in-licenses or acquires; |
• | seeks regulatory approvals for any product candidates that successfully complete clinical trials; |
• | contracts to manufacture its product candidates; |
• | establishes on its own or with partners, a sales, marketing and distribution infrastructure to commercialize any products for which Ocuphire may obtain regulatory approval; |
• | maintains, expands and protects its intellectual property portfolio; |
• | hires additional staff, including clinical, scientific, operational and financial personnel, to execute its business plan; |
• | adds operational, financial and management information systems and personnel, including personnel to support its product development and potential future commercialization efforts; and |
• | operates as a public company. |
• | per patient trial costs; |
• | the number of patients that participate in the trials; |
• | the number of sites included in the trials; |
• | the countries in which the trials are conducted; |
• | the length of time required to enroll eligible patients; |
• | the number of doses that patients receive; |
• | the drop-out or discontinuation rates of patients; |
• | potential additional safety monitoring or other studies requested by regulatory agencies; |
• | the duration of patient follow-up; |
• | the phase of development of the product candidate; |
• | arrangements with contract research organizations and other service providers; and |
• | the efficacy and safety profile of the product candidates. |
| | | For the Year Ended December 31, | |||||||
| | | 2019 | | | 2018 | | | Change | |
Operating expenses: | | | | | | | |||
General and administrative | | | $1,820,477 | | | $743,279 | | | $1,077,198 |
Research and development | | | 2,372,502 | | | 555,951 | | | 1,816,551 |
Acquired in-process research and development | | | — | | | — | | | — |
Total operating expenses | | | 4,192,979 | | | 1,299,230 | | | 2,893,749 |
Loss from operations | | | (4,192,979) | | | (1,299,230) | | | (2,893,749) |
Interest expense, net | | | (1,409,096) | | | (196,506) | | | (1,212,590) |
Fair value change of premium conversion derivatives | | | (499,414) | | | (21,238) | | | (478,176) |
Gain on note extinguishment | | | — | | | — | | | — |
Other (expense) income, net | | | (67,471) | | | (109,897) | | | 42,426 |
Loss before income taxes | | | (6,168,960) | | | (1,626,871) | | | (4,542,089) |
Provision for income taxes | | | — | | | — | | | — |
Net loss | | | $(6,168,960) | | | $(1,626,871) | | | $(4,542,089) |
| | | For the Three Months Ended June 30, | | | For the Six Months Ended June 30, | |||||||||||||
| | | 2020 | | | 2019 | | | Change | | | 2020 | | | 2019 | | | Change | |
Operating expenses: | | | | | | | | | | | | | ||||||
General and administrative | | | $551,391 | | | $414,061 | | | $137,330 | | | $942,471 | | | $777,189 | | | $165,282 |
Research and development | | | 710,752 | | | 450,885 | | | 259,867 | | | 928,561 | | | 828,450 | | | 100,111 |
Acquired in-process research and development | | | — | | | — | | | — | | | 2,126,253 | | | — | | | 2,126,253 |
Total operating expenses | | | 1,262,143 | | | 864,946 | | | 397,197 | | | 3,997,285 | | | 1,605,639 | | | 2,391,646 |
Loss from operations | | | (1,262,143) | | | (864,946) | | | (397,197) | | | (3,997,285) | | | (1,605,639) | | | (2,391,646) |
Interest expense, net | | | (688,865) | | | (213,928) | | | (474,937) | | | (1,242,624) | | | (319,869) | | | (922,755) |
Fair value change of premium conversion derivatives | | | (919,409) | | | (34,653) | | | (884,756) | | | (721,444) | | | (132,083) | | | (589,361) |
Gain on note extinguishment | | | 1,260,350 | | | — | | | 1,260,350 | | | 1,260,350 | | | — | | | 1,260,350 |
Other (expense) income, net | | | 5,885 | | | — | | | 5,885 | | | 8,505 | | | — | | | 8,505 |
Loss before income taxes | | | (1,604,182) | | | (1,113,527) | | | (490,655) | | | (4,692,498) | | | (2,057,591) | | | (2,634,907) |
Provision for income taxes | | | — | | | — | | | — | | | — | | | — | | | — |
Net loss | | | $(1,604,182) | | | $(1,113,527) | | | $(490,655) | | | $(4,692,498) | | | $(2,057,591) | | | $(2,634,907) |
• | continues clinical trials for Nyxol, APX 3330 and for any other product candidate in its future pipeline; |
• | continues preclinical studies for Nyxol, APX3330 and for any other product candidate in its future pipeline; |
• | develops additional product candidates that it identifies, in-licenses or acquires; |
• | seeks regulatory approvals for any product candidates that successfully complete clinical trials; |
• | contracts to manufacture its product candidates; |
• | establishes on its own or with partners, a sales, marketing and distribution infrastructure to commercialize any products for which it may obtain regulatory approval; |
• | maintains, expands and protects its intellectual property portfolio; |
• | hires additional staff, including clinical, scientific, operational and financial personnel, to execute its business plan; |
• | adds operational, financial and management information systems and personnel, including personnel to support its product development and potential future commercialization efforts; and |
• | operates as a public company. |
• | Qualified Financing or IPO: An amount of shares of Ocuphire common stock equal to 135% of the Note Value divided by the per share price of Ocuphire common stock issued to purchasers in the Qualified Financing or IPO. |
• | CIC: An amount of shares of Ocuphire common stock equal to 200% of the Note Value divided by the per share price of Ocuphire common stock based on the valuation of such CIC. |
• | Reverse Merger: Either (i) shares of Ocuphire common stock issued in the Reverse Merger or (ii) equity securities of the Reverse Merger counterparty, in an amount equal to 135% of the Note Value divided by the per share price at which such shares were issued to either stockholders of Ocuphire or stockholders of the Reverse Merger counterparty. |
• | IPO: An amount of shares of Ocuphire common stock equal to the greater of: (i) 150% of the Note Value divided by the per share price of Ocuphire common stock issued to purchasers in the IPO, and (ii) 100% of the Note Value divided by the per share price of $10.37. |
• | CIC: An amount of shares of Ocuphire common stock equal to the greater of: (i) 200% of the Note Value divided by the per share price of Ocuphire common stock based on the valuation of such CIC, and (ii) 100% of the Note Value divided by the per share price of $10.37. |
• | Qualified Financing: An amount of shares of Ocuphire common stock equal to 150% of the Note Value divided by the per share price of Ocuphire common stock issued to purchasers in the Qualified Financing. |
• | Reverse Merger: Either shares of Ocuphire common stock issued in the Reverse Merger or equity securities of the Reverse Merger counterparty, in an amount equal to the greater of: (i) 150% of the Note Value divided by the per share price at which such shares were issued to either stockholders of Ocuphire or stockholders of the Reverse Merger counterparty, and (ii) 100% Note Value divided by the per share price of $10.37. |
| | | Year Ended December 31, | | | Six Months Ended June 30, | |||||||
| | | 2019 | | | 2018 | | | 2020 | | | 2019 | |
Net cash used in operating activities | | | $(3,593,330) | | | $(765,367) | | | $(1,383,301) | | | $(1,313,710) |
Net cash used in investing activities | | | (24,937) | | | — | | | — | | | (20,175) |
Net cash provided by financing activities | | | 4,703,842 | | | 1,216,406 | | | 700,715 | | | 2,074,337 |
Net increase (decrease) in cash | | | $1,085,575 | | | $451,039 | | | $(682,586) | | | $740,452 |
| | | Payment Period | | | |||||||||||
| | | Less than 1 year | | | 1-3 Years | | | 3-5 Years | | | More than 5 years | | | Total | |
Facility | | | $17,850 | | | $— | | | $— | | | $ — | | | $17,850 |
Convertible notes (principal and Interest) | | | 9,129,033 | | | — | | | — | | | — | | | 9,129,033 |
Total | | | $9,146,883 | | | $— | | | $— | | | $— | | | $9,146,883 |
• | Fair Value of Common Stock. As discussed below in “—Common Stock Valuation,” because there is no public market for Ocuphire common stock as it is a private company, the Ocuphire Board has determined the fair value of the common stock by considering a number of objective and subjective factors, including based on contemporaneous valuations of Ocuphire common stock performed by an unrelated valuation specialist. The fair value of Ocuphire common stock will be approved by the Ocuphire Board until such time as its common stock is listed on an established stock exchange. |
• | Expected Term. The expected term represents the period that stock-based awards are expected to be outstanding. The expected term for option grants is determined using the simplified method where appropriate, or based on the contract term of the award . The simplified method deems the term to be the average of the time-to-vesting and the contractual life of the stock-based awards. |
• | Expected Volatility. Since Ocuphire does not have a trading history of its common stock, the expected volatility was derived from the historical stock volatilities of comparable peer public companies within its industry that Ocuphire considered to be comparable to its business over a period equivalent to the expected term of the stock-based awards. |
• | Risk-Free Interest Rate. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the date of grant for zero-coupon U.S. Treasury notes with maturities approximately equal to the stock-based awards’ expected term. |
• | Expected Dividend Rate. The expected dividend is zero as Ocuphire has not paid and does not anticipate paying any dividends for the foreseeable future. |
| | | Year Ended December 31, | | | Six Months Ended June 30, | |||||||
| | | 2019 | | | 2018 | | | 2020 | | | 2019 | |
Expected stock price volatility | | | 92.1% | | | 84.7% | | | 95.5% | | | 85.3% |
Expected life of options (years) | | | 5.5 | | | 5.0 | | | 10.0 | | | 5.2 |
Expected dividend yield | | | 0% | | | 0% | | | 0% | | | 0% |
Risk free interest rate | | | 1.7% | | | 2.9% | | | 0.7% | | | 2.1% |
• | Option pricing method (OPM). Under the option pricing method, shares are valued by creating a series of call options with exercise prices based on the liquidation preferences and conversion terms of each equity class. The values of the preferred and common stock are inferred by analyzing these options. |
• | Probability-weighted expected return method (PWERM). The PWERM is a scenario-based analysis that estimates the value per share based on the probability-weighted present value of expected future investment returns, considering each of the possible outcomes available to Ocuphire, as well as the economic and control rights of each share class. |
| | | Year Ended December 31, | | | Six Months Ended June 30, | |||||||
| | | 2019 | | | 2018 | | | 2020 | | | 2019 | |
Stock options | | | 982,219 | | | 433,719 | | | 1,175,000 | | | 481,219 |
Restricted stock awards | | | — | | | 61,100 | | | — | | | — |
Name | | | Age | | | Position |
Executive Officers | | | | | ||
Mina Sooch | | | 52 | | | President, Chief Executive Officer, Treasurer, Director, Vice Chair |
Bernhard Hoffmann | | | 65 | | | VP of Corporate Development & Finance, Secretary |
Non-Employee Directors | | | | | ||
Sean Ainsworth | | | 52 | | | Director, Lead Independent Director |
James S. Manuso | | | 72 | | | Director |
Cam Gallagher | | | 51 | | | Director, Chair of the Board |
Alan R. Meyer | | | 67 | | | Director |
Richard J. Rodgers | | | 53 | | | Director |
Susan K. Benton | | | 56 | | | Director |
• | appointing or replacing and overseeing Rexahn’s independent auditors and approving all audit engagement fees and terms; |
• | preapproving all audit (including audit-related) services, internal control-related services and permitted non-audit services (including fees and terms thereof) to be performed for Rexahn by its independent auditors; |
• | reviewing and discussing with Rexahn management and independent auditors’ significant issues regarding accounting and auditing principles and practices and financial statement presentations; |
• | reviewing and approving Rexahn’s procedures for the receipt, retention and treatment of complaints regarding accounting, internal accounting controls or auditing matters and the confidential, anonymous submission by Rexahn employees of concerns regarding accounting or auditing matters; and |
• | reviewing and overseeing Rexahn’s compliance with legal and regulatory requirements. |
• | fixing salaries of executive officers and reviewing salary plans for other executives in senior management positions; |
• | reviewing and making recommendations with respect to the compensation and benefits for Rexahn’s non-employee directors, including through equity-based plans; |
• | evaluating the performance of Rexahn’s chief executive officer and other senior executives and assisting the Rexahn Board in developing and evaluating potential candidates for executive positions; and |
• | administering the incentive compensation, deferred compensation and equity-based plans pursuant to the terms of the respective plans. |
• | reviewing, evaluating and seeking out candidates qualified to become members of the Rexahn Board; |
• | reviewing committee structure and recommending directors for appointment to committees; |
• | developing, reevaluating (not less frequently than every three years) and recommending the selection criteria for board and committee membership; |
• | establishing procedures to oversee evaluation of the board, its committees, individual directors and management; and |
• | developing and recommending guidelines on corporate governance. |
Name | | | Option Awards ($)(1)(2) | | | All Other Compensation ($) | | | Total ($) |
Sean Ainsworth | | | $47,409 | | | — | | | $47,409 |
James S. Manuso, Ph.D. | | | $47,409 | | | — | | | $47,409 |
Cam Gallagher | | | $47,409 | | | — | | | $47,409 |
Alan R. Meyer | | | $28,033 | | | $34,138 | | | $62,171 |
(1) | The amounts reported do not reflect the amounts actually received by Ocuphire’s non-employee directors. Instead, these amounts reflect the aggregate grant date fair value of each equity award granted to Ocuphire’s non-employee directors during the fiscal year ended December 31, 2019, as computed in accordance with ASC 718. Assumptions used in the calculation of these amounts are included in Note 7 to Ocuphire’s financial statements included in this prospectus/proxy statement/information statement. As required by SEC rules, the amounts shown exclude the impact of estimated forfeitures related to service-based vesting conditions. |
(2) | Reflects options to purchase 180,000 shares of Ocuphire common stock held by each director as of December 31, 2019. Each director received these options upon appointment as a director granted under the Ocuphire 2018 Plan. The options vest monthly over a 24- to 36-month period commencing in December 2019. The amounts reported represent the grant date fair value of the stock options. |
| | | Member Annual Service Stipend(1) | | | Chairperson Annual Service Stipend(1) | |
Board of directors | | | $40,000 | | | $35,000 |
Audit committee | | | 7,500 | | | 15,000 |
Compensation committee | | | 5,000 | | | 10,000 |
Nominating and corporate governance committee | | | 4,000 | | | 8,000 |
Lead Independent Director | | | 20,000 | | | — |
(1) | Chairs will not receive a stipend for being a member of the applicable committee. |
• | Mina Sooch, President, Chief Executive Officer, and Treasurer; and |
• | Bernhard Hoffmann, Vice President of Corporate Development and Finance. |
NAME AND PRINCIPAL POSITION | | | YEAR | | | SALARY ($) | | | BONUS ($) | | | OPTION AWARDS ($)(1) | | | ALL OTHER COMPENSATION ($)(2) | | | TOTAL ($) |
Mina Sooch | | | 2019 | | | 400,000 | | | 200,000 | | | 140,164 | | | 22,052 | | | 762,216 |
President, Chief Executive Officer and Treasurer | | | 2018 | | | 66,667(3) | | | — | | | 109,025 | | | 10,575 | | | 119,600 |
| | | | | | | | | | | | | |||||||
Bernhard Hoffmann | | | 2019 | | | 114,000 | | | 43,000 | | | 26,631 | | | 10,413 | | | 224,044 |
Vice President of Corporate Development and Finance, Secretary | | | 2018 | | | 24,000(4) | | | — | | | 26,099 | | | 11,801 | | | 97,900 |
(1) | The amounts reported reflect the aggregate grant date fair value of the stock options granted to Ocuphire’s named executive officers during 2019 and 2018. Assumptions used in the calculation of these amounts are included in Note 7 to Ocuphire’s audited financial statements included elsewhere in this proxy statement/prospectus/information statement. As required by SEC rules, the amounts shown exclude the impact of estimated forfeitures related to service-based vesting conditions. |
(2) | In 2018, Ms. Sooch and Mr. Hoffmann received cash payments of $10,000 and $11,000, respectively, in connection with the execution of their employment agreements. Unless otherwise noted, all other amounts reflect the dollar value of group health insurance premiums paid during 2018 and 2019 with respect to health insurance coverage for the named executive officer. |
(3) | Pursuant to Ms. Sooch’s employment agreement, 50% of such salary amounts were deferred in 2018 and paid out in cash in 2019. |
(4) | Pursuant to Mr. Hoffmann’s employment agreement, 50% of such salary amounts were deferred in 2018 and paid out in cash in 2019. |
Name | | | Number of securities underlying unexercised options exercisable | | | Number of securities underlying unexercised options unexercisable | | | Option Exercise price ($) | | | Option Grant Date | | | Option Expiration date |
Mina Sooch | | | 168,750 | | | — | | | 0.95 | | | 10/1/2018 | | | 10/1/2028 |
| | 18,000 | | | 132,000(1) | | | $1.27 | | | 12/27/2019 | | | 12/27/2029 | ||
Bernhard Hoffmann | | | 40,575 | | | — | | | 0.95 | | | 10/1/2018 | | | 10/1/2028 |
| | 3,300 | | | 25,200(2) | | | $1.27 | | | 12/27/2019 | | | 12/27/2029 |
(1) | 18,000 shares vested on December 31, 2019, and the remaining shares vest in equal monthly installments from January 2020 through December 2021, subject to continued service. |
(2) | 3,300 shares vested on December 31, 2019, and the remaining shares vest in equal monthly installments from January 2020 through December 2021, subject to continued service. |
• | “termination for cause” means a termination of Ms. Sooch’s employment by Ocuphire due to (i) acts of dishonesty undertaken by Ms. Sooch and intended to result in personal enrichment to her at the expense of Ocuphire; (ii) gross misconduct on the part of Ms. Sooch that is injurious to Ocuphire; (iii) Ms. Sooch’s commission of, or entry into a no contest plea to, any felony; (iv) breach by Ms. Sooch of her fiduciary obligations as an officer or director of Ocuphire; (v) a persistent and deliberate failure by Ms. Sooch to perform the duties and responsibilities of her employment which remains uncured for 30 days after Ocuphire provides Ms. Sooch with written notice of her intentional action or conduct; or (vi) material breach of any terms and conditions of her employment agreement which remains uncured for 10 days after Ocuphire provides Ms. Sooch with written notice. |
• | “termination for good reason” means a termination of Ms. Sooch’s employment by Ms. Sooch within 30 days of Ocuphire’s failure to cure any of the following: (i) a material reduction in her base salary (unless such reduction is pursuant to a salary reduction program applicable generally to Ocuphire’s similarly situated executives); (ii) removal of Ms. Sooch by Ocuphire from the position of President and Chief Executive Officer; (iii) a material reduction in Ms. Sooch’s authority, duties or |
• | “termination for cause” means a termination of Mr. Hoffmann’s employment by Ocuphire due to (i) acts of dishonesty undertaken by Mr. Hoffmann and intended to result in personal enrichment to her at the expense of Ocuphire; (ii) gross misconduct on the part of Mr. Hoffmann that is injurious to Ocuphire; (iii) Mr. Hoffmann’s commission of, or entry into a no contest plea to, any felony; (iv) breach by Mr. Hoffmann of his fiduciary obligations as an officer or director of Ocuphire; (v) a persistent and deliberate failure by Mr. Hoffmann to perform the duties and responsibilities of his employment which remains uncured for 30 days after Ocuphire provides Mr. Hoffmann with written notice of his intentional action or conduct; or (vi) material breach of any terms and conditions of his employment agreement which remains uncured for 10 days after Ocuphire provides Mr. Hoffmann with written notice. |
• | “termination for good reason” means a termination of Mr. Hoffmann’s employment by Mr. Hoffmann within 30 days of Ocuphire’s failure to cure any of the following: (i) a material reduction in his base salary (unless such reduction is pursuant to a salary reduction program applicable generally to Mr. Hoffmann’s similarly situated executives); (ii) removal of Mr. Hoffmann by Ocuphire from the position of Vice President; (iii) a material reduction in Mr. Hoffmann’s authority, duties or responsibilities; (iv) a material change in Mr. Hoffmann’s reporting relationships; or (v) a material breach by Ocuphire of any material provision of Mr. Hoffmann’s employment agreement. |
Name and Principal Position(s) | | | Year | | | Salary ($) | | | Bonus ($) | | | Option Awards ($)(1)(2) | | | Non- Equity Incentive Plan ($) | | | All Other Compensation ($) | | | Total ($) |
Douglas J. Swirsky President and Chief Executive Officer | | | 2019 | | | 425,000 | | | — | | | — | | | 212,500 | | | 13,177 | | | 650,677 |
| | 2018 | | | 359,952 | | | — | | | 703,977 | | | 106,250 | | | 14,175 | | | 1,184,354 | ||
Ely Benaim(3) Former Chief Medical Officer | | | 2019 | | | 110,000 | | | 88,000 | | | 41,254 | | | — | | | 5,775 | | | 245,029 |
| | 2018 | | | 440,000 | | | — | | | 294,861 | | | 100,000 | | | 16,500 | | | 851,361 | ||
Lisa Nolan(3) Former Chief Business Officer | | | 2019 | | | 262,500 | | | — | | | 53,630 | | | — | | | 59,556 | | | 375,686 |
| | 2018 | | | 335,000 | | | — | | | 62,999 | | | 80,000 | | | 16,407 | | | 494,406 |
(1) | Reflects grant date fair value computed in accordance with ASC 718. A discussion of assumptions used in calculating grant date fair value of Rexahn’s equity awards can be found in Note 11 to Rexahn’s financial statements included in this proxy statement/prospectus/information statement. |
(2) | The actual value realized by each officer with respect to option awards will depend on the difference between the market value of Rexahn common stock on the date the option is exercised and the exercise price. |
(3) | Dr. Benaim resigned from Rexahn in March 2019, and Dr. Nolan resigned from Rexahn in September 2019. For Dr. Nolan, All Other Compensation for 2019 includes $45,000 in payments for post-resignation consulting services. |
• | a reduction of his salary or target bonus percentage; |
• | a relocation requiring him to be based at any office that is more than 35 miles from Rexahn’s office at the time of the signing of the agreement; |
• | any material breach by Rexahn of the terms and provisions of the agreement or any other material agreement between Mr. Swirsky and Rexahn; or |
• | a material diminution in his duties or authority inconsistent with his position. |
| | | Number of Securities Underlying Unexercised Options (#) | | | | | ||||||
Name | | | Exercisable | | | Unexercisable | | | Option Exercise Price ($) | | | Option Expiration Date |
Douglas J. Swirsky | | | 9,982(1) | | | 10,851(1) | | | 25.20 | | | 1/2/2028 |
| | | 11,284(2) | | | 30,382(2) | | | 13.08 | | | 11/14/2028 | |
Ely Benaim | | | — | | | — | | | — | | | — |
Lisa Nolan | | | — | | | — | | | — | | | — |
(1) | Represents option award granted under the 2013 Plan on January 2, 2018, which vested 25% on January 2, 2019, and 1/48th of which vested or will vest on the first business day of each month beginning in February 2019 and ending in January 2022. |
(2) | Represents option award granted under the 2013 Plan on November 14, 2018, which vested 25% on November 14, 2019, and 1/48th of which vested or will vest on the first business day of each month beginning in December 2019 and ending in November 2022. |
Position | | | Compensation* |
Director | | | $40,000 per annum, plus an additional $25,000 for the Chairman of the Board |
Audit Committee (Chair) | | | $15,000 per annum |
Audit Committee (Member) | | | $7,500 per annum |
Compensation Committee (Chair) | | | $10,000 per annum |
Compensation Committee (Member) | | | $5,000 per annum |
Nominating and Corporate Governance Committee (Chair) | | | $7,500 per annum |
Nominating and Corporate Governance Committee (Member) | | | $3,750 per annum |
Business Development Committee (Chair) | | | $10,000 per annum |
Business Development Committee (Member) | | | $5,000 per annum |
* | Paid semi-annually. |
Name | | | Fees Earned Or Paid In Cash ($) | | | Option Awards ($)(1) | | | Total ($) |
Peter Brandt | | | 82,500 | | | 11,851 | | | 94,351 |
Charles Beever | | | 58,090 | | | 11,851 | | | 69,941 |
Kwang Soo Cheong | | | 51,250 | | | 11,851 | | | 63,101 |
Richard J. Rodgers | | | 60,000 | | | 11,851 | | | 71,851 |
Ben Gil Price | | | 49,803 | | | 11,851 | | | 61,654 |
Lara Sullivan(2) | | | 45,287 | | | 41,651 | | | 86,938 |
(1) | Grant date fair value computed in accordance with ASC 718. The actual value realized with respect to option awards will depend on the difference between the market value of Rexahn common stock on the date the option is exercised and the exercise price. As of December 31, 2019, Mr. Beever, Dr. Cheong and Mr. Brandt each had 11,887 option awards outstanding; Mr. Rodgers had 10,639 option awards outstanding; Dr. Price had 12,943 option awards outstanding; and Dr. Sullivan had 10,617 option awards outstanding. |
(2) | Dr. Sullivan joined the Rexahn Board in February 2019. |
• | the amounts involved exceeded or will exceed the lesser of $120,000 or 1% of the average of the total assets of Rexahn or Ocuphire, as the case may be, at year-end for the last two completed fiscal years; and |
• | a director, executive officer, holder of more than 5% of the outstanding capital stock of Rexahn, Ocuphire or the combined company or any member of such person’s immediate family had or will have a direct or indirect material interest. |
Name of Noteholder | | | Principal Amount of Convertible Notes ($) |
Mina Sooch | | | 200,540 |
Bernhard Hoffmann | | | 22,500 |
Sean Ainsworth | | | 100,000 |
Cam Gallaher | | | 100,000 |
James S. Manuso | | | 75,000 |
Alan R. Meyer | | | 243,982 |
| | | Rexahn | | | Ocuphire | | | Pro Forma Adjustments | | | Notes | | | Pro Forma Combined | |
Assets | | | | | | | | | | | |||||
Current assets: | | | | | | | | | | | |||||
Cash and cash equivalents | | | $9,208,951 | | | $854,331 | | | $19,058,000 | | | I, L | | | $29,121,282 |
Proceeds receivable from convertible notes | | | — | | | 1,425,000 | | | (1,425,000) | | | H | | | — |
Prepaid expenses and other current assets | | | 817,653 | | | 23,439 | | | 1,425,000 | | | H | | | 2,266,092 |
Deferred offering costs | | | — | | | 1,181,334 | | | (1,181,334) | | | K | | | — |
Total current assets | | | 10,026,604 | | | 3,484,104 | | | 17,876,666 | | | | | 31,387,374 | |
Property and equipment, net | | | 57,312 | | | 15,804 | | | (34,203) | | | J | | | 38,913 |
Right-of-use assets | | | 139,477 | | | — | | | (139,477) | | | J | | | — |
Deposits | | | 25,681 | | | — | | | — | | | | | 25,681 | |
Total assets | | | $10,249,074 | | | $3,499,908 | | | $17,702,986 | | | | | $31,451,968 | |
Liabilities and Stockholders' Equity (Deficit) | | | | | | | | | | | |||||
Current liabilities: | | | | | | | | | | | |||||
Accounts payable and accrued expenses | | | $2,190,753 | | | $1,976,668 | | | $4,785,596 | | | F, G | | | $8,953,017 |
Deferred revenue | | | 650,000 | | | — | | | (650,000) | | | J | | | — |
Convertible notes | | | — | | | 9,120,828 | | | (9,120,828) | | | A, B | | | — |
Premium conversion derivative | | | — | | | 1,179,765 | | | (1,179,765) | | | A | | | — |
Operating lease liabilities | | | 136,197 | | | — | | | — | | | | | 136,197 | |
Total current liabilities | | | 2,976,950 | | | 12,277,261 | | | (6,164,997) | | | | | 9,089,214 | |
Warrant liabilities | | | 268,811 | | | — | | | (194,278) | | | E | | | 74,533 |
Total liabilities | | | 3,245,761 | | | 12,277,261 | | | (6,359,275) | | | | | 9,163,747 | |
Stockholders’ Equity (Deficit): | | | | | | | | | | | |||||
Preferred stock | | | — | | | — | | | — | | | | | — | |
Common stock | | | 402 | | | 354 | | | 1,856 | | | O | | | 2,612 |
Additional paid-in capital | | | 173,423,515 | | | 3,969,494 | | | (125,154,801) | | | M | | | 52,238,208 |
Accumulated deficit | | | (166,420,604) | | | (12,747,201) | | | 149,215,206 | | | N | | | (29,952,599) |
Total stockholders' equity (deficit) | | | 7,003,313 | | | (8,777,353) | | | 24,062,261 | | | | | 22,288,221 | |
Total liabilities and stockholders' equity (deficit) | | | $10,249,074 | | | $3,499,908 | | | $17,702,986 | | | | | $31,451,968 |
| | | Rexahn | | | Ocuphire | | | Pro Forma Adjustments | | | Notes | | | Pro Forma Combined | |
Revenues: | | | | | | | | | | | |||||
Revenue | | | $1,150,000 | | | $— | | | $— | | | | | $1,150,000 | |
Total revenues | | | 1,150,000 | | | — | | | — | | | | | 1,150,000 | |
Operating expenses: | | | | | | | | | | | |||||
General and administrative | | | 3,372,898 | | | 942,471 | | | (1,610,314) | | | Q, S | | | 2,705,055 |
Research and development | | | 688,397 | | | 928,561 | | | — | | | | | 1,616,958 | |
Acquired in-process research and development | | | — | | | 2,126,253 | | | — | | | | | 2,126,253 | |
Total operating expenses | | | 4,061,295 | | | 3,997,285 | | | (1,610,314) | | | | | 6,448,266 | |
Loss from operations | | | (2,911,295) | | | (3,997,285) | | | 1,610,314 | | | | | (5,298,266) | |
Interest expense | | | — | | | (1,242,624) | | | 1,242,624 | | | R | | | — |
Fair value change in warrant liability and premium conversion derivative | | | (227,094) | | | (721,444) | | | 721,444 | | | R | | | (227,094) |
Gain on note extinguishment | | | — | | | 1,260,350 | | | (1,260,350) | | | R | | | — |
Interest income | | | 40,461 | | | 8,505 | | | — | | | | | 48,966 | |
Net loss | | | $(3,097,928) | | | $(4,692,498) | | | $2,314,032 | | | | | $(5,476,394) | |
Net loss per share, basic and diluted | | | $(0.77) | | | $(1.36) | | | | | | | $(0.21) | ||
Weighted average common shares outstanding, basic and diluted | | | 4,019,141 | | | 3,451,031 | | | 18,287,540 | | | P | | | 25,757,712 |
| | | Rexahn | | | Ocuphire | | | Pro Forma Adjustments | | | Notes | | | Pro Forma Combined | |
Revenues: | | | | | | | | | | | |||||
Revenue | | | $— | | | $— | | | $— | | | | | $— | |
Total revenues | | | — | | | — | | | — | | | | | — | |
Operating expenses: | | | | | | | | | | | |||||
General and administrative | | | 5,738,227 | | | 1,820,477 | | | (488,069) | | | Q, S | | | 7,070,635 |
Research and development | | | 5,476,776 | | | 2,372,502 | | | — | | | | | 7,849,278 | |
Acquired in-process research and development | | | — | | | — | | | — | | | | | — | |
Total operating expenses | | | 11,215,003 | | | 4,192,979 | | | (488,069) | | | | | 14,919,913 | |
Loss from operations | | | (11,215,003) | | | (4,192,979) | | | 488,069 | | | | | (14,919,913) | |
Interest expense | | | — | | | (1,409,096) | | | 1,409,096 | | | R | | | — |
Fair value change in warrant liability and premium conversion derivative | | | 2,265,869 | | | (499,414) | | | 499,414 | | | R | | | 2,265,869 |
Interest income | | | 313,700 | | | 510 | | | — | | | | | 314,210 | |
Other | | | — | | | (67,981) | | | — | | | | | (67,981) | |
Net loss | | | $(8,635,434) | | | $(6,168,960) | | | $2,396,579 | | | | | $(12,407,815) | |
Net loss per share, basic and diluted | | | $(2.18) | | | $(2.29) | | | — | | | | | $(0.59) | |
Weighted average common shares outstanding, basic and diluted | | | 3,960,163 | | | 2,692,793 | | | 14,427,711 | | | P | | | 21,080,667 |
1. | Description of Transaction |
2. | Estimated Purchase Price |
Estimated number of shares of the combined company to be owned by Rexahn's stockholders(i) | | | 4,019,141 |
Multiplied by the fair value per share of Rexahn's common stock(ii) | | | $2.84 |
Total | | | $11,414,360 |
Rexahn warrants assumed in merger | | | 1,496,875 |
Rexahn stock options assumed in merger | | | 344 |
Estimated transaction costs | | | 1,628,363 |
Total estimated purchase price | | | $14,539,942 |
Net assets as of June 30, 2020 | | | $7,748,444 |
In process research and development(iii) | | | 6,791,498 |
Total estimated purchase price | | | $14,539,942 |
(i) | The final purchase price will be determined based in part on the number of shares of Rexahn common stock and the value of Rexahn Warrants and Rexahn Options outstanding immediately prior to the merger. For purposes of this unaudited pro forma condensed combined financial information, the estimated number of shares represents 4,019,141 shares of Rexahn common stock outstanding as of June 30, 2020. The estimated number of shares does not reflect the impact of the Rexahn Reverse Stock Split that is expected to be effected prior to consummation of the merger. |
(ii) | The estimated purchase price was based on the Rexahn June 30, 2020 closing price as reported on the Nasdaq Capital Market. The final purchase price arising from the actual transaction costs, the number of shares and fair value of Rexahn common stock as well as the fair value of Rexahn Warrants and Rexahn Options outstanding immediately prior to the Closing could result in a total purchase price different from that assumed in this unaudited pro forma condensed combined financial information, and that difference may be material. Therefore, the estimated consideration expected to be transferred reflected in this unaudited pro forma condensed combined |
(iii) | IPR&D represents the research and development projects of Rexahn which were in-process, but not yet completed, and which Ocuphire plans to advance. This includes the development of RX-3117, RX-0301 and RX-0047. Current accounting standards require that the fair value of IPR&D projects acquired in an asset acquisition with no alternative future use be allocated a portion of the consideration transferred and charged to expense at the acquisition date. The acquired assets did not have outputs or employees. The actual purchase price allocated to IPR&D will fluctuate until the Closing, and the final valuation of the IPR&D consideration could differ significantly from the current estimate. |
3. | Pro Forma Adjustments |
A. | To reflect the conversion of the Ocuphire convertible notes (principal and accrued interest) and the application of premium conversion derivatives into shares of Rexahn common stock prior to the merger, in accordance with the terms of the Note Conversion Agreement. |
B. | To record the remaining debt discount amortization expense on the Ocuphire convertible notes. The accelerated debt discount amortization expense is not reflected in the pro forma statements of operations because it does not have a continuing impact. Adjustments to convertible notes are as follows: |
| | | June 30, 2020 | |
Conversion of Ocuphire's convertible notes principal and interest (A) | | | $(9,129,033) |
Debt discount amortization expense on Ocuphire's convertible notes payable (B) | | | 8,205 |
Total | | | $(9,120,828) |
C. | To reflect accounting treatment of Ocuphire convertible notes conversion into common stock as a loss on extinguishment in connection with the merger. The loss on extinguishment is not reflected in the pro forma statements of operations because it does not have a continuing impact. |
D. | To record the estimated fully vested fair value of Rexahn Options assumed in connection with the merger. |
E. | To reflect the estimated fair value of Rexahn Warrants assumed in connection with the merger (including both liability and equity classified portions), and assuming holders of Rexahn Warrants do not exercise their right to exchange their Rexahn Warrants for cash in an amount equal to the Black-Scholes value of such warrants calculated as set forth therein and in accordance with the terms of such Rexahn Warrants. |
F. | To record Rexahn’s estimated transaction costs, such as severance and benefits, advisory fees and transactional fees, that were not incurred as of June 30, 2020. The Rexahn transaction costs are not reflected in the pro forma statements of operations because they do not have a continuing impact. |
G. | To record Ocuphire’s estimated transaction costs, such as legal, accounting, advisory and other transactional fees, that were not incurred as of June 30, 2020. The Ocuphire transaction costs are not reflected in the pro forma statements of operations because they do not have a continuing impact. |
| | | June 30, 2020 | |
Rexahn's estimated transaction costs (F) | | | $4,338,567 |
Ocuphire's estimated transaction costs (G) | | | 447,029 |
Total | | | $4,785,596 |
H. | To reclassify proceeds receivable from convertible notes to other current assets as a result of the note conversions into Ocuphire common stock in connection with the merger. |
I. | To reflect the Pre-Merger Financing upon Closing for a total of $21.15 million in gross proceeds, less issuance costs of $1.7 million. The accounting treatment under Accounting Standards Codification (ASC) 480 – Distinguishing Liabilities from Equity and ASC 815 – Derivatives and Hedging is in process related to the Pre-Merger Financing, including the accounting classification of the Investor Warrants and Additional Shares. For purposes of these pro formas, the Pre-Merger Financing has been classified as equity. Upon closing of the Pre-Merger Financing, certain cash settlement provisions, registration requirements, or other adjustments not afforded to other stockholders, may result in the Investor Warrants and Additional Shares being accounted for as a liability on the balance sheet until all of the settlement contingencies are resolved for those instruments. The liability accounting impact would result in some of the Pre-Merger Financing, currently classified in the pro formas as equity, to be reclassified as a liability on the condensed combined balance sheet. In addition, any liability recognized for the Pre-Merger Financing would be subject to remeasurement at fair value as of each reporting period with offsetting impacts of the fair value changes to the statement of operations. |
J. | To adjust Rexahn’s historical financial statements to give pro forma effect to events in connection with the merger that include: 1) the elimination of Rexahn’s historical common stock, paid-in-capital and accumulated deficit balances; 2) the elimination of Rexahn’s deferred revenue liability given the non-assumption of the obligation post-merger; and 3) the write-down of Rexahn’s facility lease and property and equipment reported values to reflect their fair value based on their anticipated non-usage post-merger. |
K. | To reflect the following impacts to the historical financial statements to give pro forma effect to events in connection with the merger that include: 1) the expensing of Rexahn’s IPR&D; 2) the capitalization of the fair value of the estimated number of common shares, warrants and stock options of the combined company to be owned by Rexahn Stockholders; 3) the impact of transaction costs impacting the estimated purchase price of the merger; and 4) to reflect the impact of the Exchange Ratio to the outstanding common shares of the combined company. The IPR&D expense is not reflected in the pro forma statements of operations because it does not have a continuing impact. |
L. | To reflect milestone payments due to Apexian upon Closing. |
M. | Adjustments to additional-paid-in-capital are as follows: |
| | | June 30, 2020 | |
Conversion of Ocuphire's convertible notes and accrued interest (A) | | | $9,128,943 |
To reflect application of premium conversion derivatives (A) | | | 1,179,765 |
To reflect extinguishment loss on convertible notes (C) | | | 5,667,128 |
Eliminate Rexahn's pre-merger additional paid-in-capital balance (J) | | | (173,423,515) |
To reflect the fair value of Rexahn's remaining common stock post-merger (K) | | | 11,413,958 |
To reflect assumption of Rexahn warrants post-merger (E) | | | 1,422,342 |
To reflect assumption of Rexahn stock options post-merger (D) | | | 344 |
To reflect impact of Exchange Ratio to pre-merger Ocuphire shares (K) | | | (1,649) |
To reflect Ocuphire’s Pre-Merger Financing in connection with the merger (I) | | | 19,457,883 |
Total | | | $(125,154,801) |
N. | Adjustments to accumulated deficit are as follows: |
| | | June 30, 2020 | |
Debt discount amortization expense on Ocuphire’s convertible notes (B) | | | $(8,205) |
To reflect extinguishment loss on Ocuphire’s convertible notes (C) | | | (5,667,128) |
Rexahn's estimated transaction costs (F) | | | (4,338,567) |
Milestone payment to Apexian (L) | | | (400,000) |
Eliminate Rexahn's pre-merger accumulated deficit balance (J) | | | 166,420,604 |
To reflect impact of non-equity related Rexahn acquisition cost (G) (K) | | | (6,791,498) |
Total | | | $149,215,206 |
O. | Adjustments to common stock par value are as follows: |
| | | June 30, 2020 | |
Conversion of Ocuphire's convertible notes into common stock (A) | | | $90 |
To reflect Ocuphire’s Pre-Merger Financing in connection with the merger (I) | | | 117 |
Eliminate Rexahn's pre-merger common stock balance (J) | | | (402) |
To reflect impact of Exchange Ratio to pre-merger Ocuphire shares (K) | | | 1,649 |
To reflect Rexahn’s ownership in the combined company (K) | | | 402 |
Total | | | $1,856 |
P. | The pro forma combined basic and diluted net loss per share calculations have been adjusted to reflect the pro forma net loss for the six months ended June 30, 2020 and for the year ended December 31, 2019. In addition, the number of shares used in calculating the pro forma combined basic and diluted net loss per share has been adjusted to reflect the estimated total number of shares of common stock of the combined company that would be outstanding on a weighted-average basis as of the Closing of the merger. The following table is a reconciliation of each company’s historical basic and diluted loss per share to its pro forma basic and diluted loss per share for the six months ended June 30, 2020 and for the year ended December 31, 2019. |
| | | | | Six Months Ended June 30, 2020 | | | Year Ended December 31, 2019 | ||
Basic and Diluted Loss Per Share: | | | | | | | |||
As reported (Rexahn) | | | a/d | | | $(0.77) | | | $(2.18) |
As reported (Ocuphire) | | | b/e | | | $(1.36) | | | $(2.29) |
Pro forma | | | c/f | | | $(0.21) | | | $(0.59) |
| | | | | Six Months Ended June 30, 2020 | | | Year Ended December 31, 2019 | ||
Net loss: | | | | | | | |||
As reported (Rexahn) | | | a | | | $(3,097,928) | | | $(8,635,434) |
As reported (Ocuphire) | | | b | | | (4,692,498) | | | (6,168,960) |
Add: Rexahn's transaction costs expensed through the statement of operations (Q) | | | | | 1,591,856 | | | 447,077 | |
Add: Depreciation and amortization expenses associated with Rexahn operations (S) | | | | | 18,458 | | | 40,992 | |
Add: Interest expense associated with Ocuphire's convertible notes (R) | | | | | 1,242,624 | | | 1,409,096 | |
Add: Fair Value adjustment related to Ocuphire premium conversion derivatives (R) | | | | | 721,444 | | | 499,414 | |
Subtract: Gain on note extinguishment (R) | | | | | (1,260,350) | | | — | |
Pro forma | | | c | | | $(5,476,394) | | | $(12,407,815) |
Basic and Diluted Weighted Average Shares: | | | | | | | |||
As reported (Rexahn) | | | d | | | 4,019,141 | | | 3,960,163 |
As reported (Ocuphire) | | | e | | | 3,451,031 | | | 2,692,793 |
Add: Application of the estimated Exchange Ratio of 3.9386 to Ocuphire's weighted average common shares outstanding | | | | | 10,141,200 | | | 7,913,042 | |
Add: Conversion of Ocuphire's convertible notes convertible notes and accrued interest upon closing of the merger as adjusted for the Exchange Ratio of 3.9386 | | | | | 3,533,708 | | | 1,902,037 | |
Add: Closing of Ocuphire’s private placement of common stock and warrant financing contemplated by the Pre-Merger Financing upon Closing of the merger as adjusted for the Exchange Ratio of 3.9386 | | | | | 4,612,632 | | | 4,612,632 | |
Pro forma | | | f | | | 25,757,712 | | | 21,080,667 |
Q. | To reflect the elimination of Rexahn’s transaction costs expensed through the statement of operations for the year ended December 31, 2019 and for the six month period ended June 30, 2020. |
R. | To reverse interest expense and fair value of changes in premium conversion derivatives and gain on note extinguishment associated with the Ocuphire convertible notes. The pro forma income statement assumes conversion of the notes at the beginning of the period presented. |
S. | To reflect the elimination of the historical Rexahn depreciation and amortization expense in the historical period that will not have a continuing impact on the pro forma statement of operations. |
• | to cast one vote for each share held of record on all matters submitted to a vote of the stockholders; |
• | to receive dividends, as may be lawfully declared from time to time by the Rexahn Board, subject to any preferential rights of holders of any outstanding shares of preferred stock; and |
• | in the event of Rexahn’s liquidation, dissolution or winding up, whether voluntary or involuntary, after payment of Rexahn’s debts and other liabilities and making provision for the holders of outstanding shares of preferred stock, if any, to share ratably in the remainder of Rexahn’s assets. |
• | as to director nominations, all information relating to each director nominee that is required by the rules of the SEC to be disclosed in solicitations of proxies, or is otherwise required by Regulation 14A of the Exchange Act; |
• | as to any other business that the stockholder proposes to bring before the meeting, a brief description of the business to be proposed, the reasons for conducting such business at the meeting and, if any, the stockholder’s material interest in the proposed business; and |
• | the name and address of the stockholder who intends to make the nomination and the class and number of Rexahn shares beneficially owned of record; |
Provision | | | Ocuphire (Pre-Merger) | | | Rexahn (Post-Merger) |
Elections; Voting; Procedural Matters | ||||||
| | | | | |||
Authorized Capital Stock | | | The Ocuphire Certificate of Incorporation authorizes the issuance of up to 5,000,000 shares of common stock, par value $0.0001 per share, and 625,000 shares of preferred stock, par value $0.0001 per share. | | | The Rexahn Certificate of Incorporation authorizes the issuance of up to 75,000,000 shares of common stock, par value $0.0001 per share, and 10,000,000 shares of preferred stock, par value $0.0001 per share. |
| | | | | |||
Number of Directors | | | The Ocuphire Bylaws currently provide that the Ocuphire Board shall consist of one or more members and that the number of directors may be changed from time to time by resolution of the Ocuphire Board. The Stockholders Agreement, dated April 10, 2018, among Ocuphire and certain Ocuphire Stockholders (the “Ocuphire Stockholders Agreement”), provides that the initial size of the Ocuphire Board shall be three directors and may be | | | The Rexahn Bylaws provide that the number of directors that constitute the whole Rexahn Board is established by the Rexahn Board. |
Provision | | | Ocuphire (Pre-Merger) | | | Rexahn (Post-Merger) |
| | | increased to up to five directors by the then-current Ocuphire Board. | | | ||
| | | | | |||
Stockholder Nominations and Proposals | | | The Ocuphire Bylaws provide that in order for a stockholder to make a director nomination or propose business at an special meeting of stockholders, the stockholder must give timely written notice to the Ocuphire secretary, which must be received not more than 120 calendar days before and not less than 90 calendar days before the first anniversary of the date of the previous year’s special meeting (with certain adjustments if no special meeting was held the previous year or the date of the special meeting is changed by more than 30 days from the first anniversary of the preceding year’s special meeting). | | | The Rexahn Bylaws provide that in order for a stockholder to make a director nomination or propose business at an annual meeting of stockholders, the stockholder must give timely written notice to the Rexahn secretary, which must be received not more than 120 calendar days before and not less than 90 calendar days before the one year anniversary of the date of the previous year’s annual meeting (with certain adjustments if the date of the annual meeting is more than 30 days before or more than 60 days after the first anniversary of the preceding year’s annual meeting). |
| | | | | |||
Classified Board of Directors | | | Neither the Ocuphire Certificate of Incorporation nor the Ocuphire Bylaws provides for the division of the Ocuphire Board into staggered classes. | | | The Rexahn Bylaws do not provide for the division of the Rexahn Board into staggered classes. |
| | | | | |||
Removal of Directors | | | The Ocuphire Bylaws provide that directors shall hold office for a term of one year and until their successors are duly elected and qualified, subject to their earlier death, resignation or removal. Any director may be removed from office at any time (i) with cause by the affirmative vote of the holders of a majority of the voting power of all then-outstanding shares of Ocuphire capital stock entitled to vote generally at an election of directors, or (ii) without cause by the affirmative vote of the holders of a majority of the voting power of all then-outstanding shares of Ocuphire capital stock, entitled to vote generally at an election of directors. Any director may resign at any time upon written notice to Ocuphire. | | | The Rexahn Certificate of Incorporation provides for the removal of any of its directors only for cause and requires a stockholder vote of at least a majority of the voting power of the then outstanding voting stock. |
| | | | | |||
Special Meetings | | | The Ocuphire Bylaws provide that special meetings of stockholders | | | The Rexahn Certificate of Incorporation and the Rexahn |
Provision | | | Ocuphire (Pre-Merger) | | | Rexahn (Post-Merger) |
| | | may be called by the Chairman of the Board, the Chief Executive Officer, the Ocuphire Board or the holders of shares entitled to case not less than 20% of the votes at the meeting. | | | Bylaws provide that a special meeting of stockholders may be called only by a resolution adopted by a majority of the board of directors or by the chairman of the board. | |
| | | | | |||
Cumulative Voting | | | The Ocuphire Certificate of Incorporation and Ocuphire Bylaws do not have a provision granting cumulative voting rights in the election of its directors. | | | The Rexahn Certificate of Incorporation and the Rexahn Bylaws do not have a provision granting cumulative voting rights in the election of its directors, unless so required by applicable law. |
| | | | | |||
Vacancies | | | The Ocuphire Bylaws provide that any vacancies on the Ocuphire Board resulting from death, resignation, disqualification, removal or other causes and any newly created directorships resulting from any increase in the number of directors shall, unless the Ocuphire Board determines by resolution that any such vacancies or newly created directorships shall be filled by stockholders, be filled only by the affirmative vote of a majority of the directors then in office, even though less than a quorum of the Ocuphire Board, or by a sole remaining director. | | | The Rexahn Certificate of Incorporation provides that any vacancy occurring on the Rexahn Board may be filled by a majority of directors then in office, even if less than a quorum, unless the board of directors determines that such vacancy shall be filled by the stockholders. |
| | | | | |||
Voting Stock | | | Under the Ocuphire Certificate of Incorporation and the Ocuphire Bylaws, the holders of voting stock are entitled to vote on each matter properly submitted to the stockholders at a meeting of the stockholders and are entitled to cast one vote in person or by proxy for each share of voting stock held by them respectively as of the record date fixed by the secretary at least 10 days before the meeting of the stockholders. | | | Under the Rexahn Certificate of Incorporation and the Rexahn Bylaws, each stockholder shall at every meeting of the stockholders be entitled to one vote for each share of stock held by such stockholder. |
| | | | | |||
Stockholder Action by Written Consent | | | The Ocuphire Bylaws provide that any action required or permitted to be taken at any special or special meeting of stockholders may be taken without a meeting, without prior notice and without a vote, if a consent in writing (or by electronic | | | The Rexahn Certificate of Incorporation provides that any action required or permitted to be taken by the stockholders may be taken by consent in writing by holders of at least a majority of the voting power of the outstanding |
Provision | | | Ocuphire (Pre-Merger) | | | Rexahn (Post-Merger) |
| | | transmission), setting forth the action so taken, is signed by the holders of outstanding stock having not less than the number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote on such action were present and voted. | | | shares of voting stock, voting together as a single class. | |
| | | | | |||
Notice of Stockholder Meeting | | | The Ocuphire Bylaws provide that written notice of all meetings of stockholders must be given, stating the place, if any, date and hour, in the case of special meetings the purpose or purposes of the meeting, and the means of remote communications, if any, by which stockholders and proxyholders may be deemed to be present in person and vote at any such meeting. The Ocuphire Bylaws provide that notice of each meeting of stockholders must be given not less than 10 nor more than 60 days before the date of the meeting to each stockholder of record entitled to vote at such meeting. | | | Under the Rexahn Bylaws, written notice of each stockholder meeting must specify the place, date and hour of the meeting, the means of remote communication, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at such meeting, and, in the case of a special meeting, the purposes for which the meeting is called. Notice shall be given not less than 10 nor more than 60 calendar days before the date of the meeting to each stockholder entitled to vote at such meeting. |
| | | | | |||
Conversion Rights and Protective Provisions | | | The Ocuphire Certificate of Incorporation does not provide that holders of Ocuphire’s capital stock have preemptive, conversion or other protective rights. | | | The Rexahn Certificate of Incorporation does not provide that holders of Rexahn capital stock have preemptive, conversion or other protective rights. |
| | | | | |||
Right of First Refusal | | | The Ocuphire Bylaws provide that any Ocuphire Stockholder wishing to transfer any shares of Ocuphire common stock must first provide Ocuphire (or any assignee) with the right to purchase such shares. The right of first refusal will terminate upon the Closing. Additionally, as further described in the Ocuphire Stockholders Agreement, a holder of 5% or more of Ocuphire capital stock (an “Ocuphire Major Shareholder”) wishing to transfer any shares of Ocuphire common stock shall first provide Ocuphire and other Ocuphire Major Shareholders with | | | Rexahn does not have a right of first refusal in place. |
Provision | | | Ocuphire (Pre-Merger) | | | Rexahn (Post-Merger) |
| | | the right to purchase such shares. The Ocuphire Stockholders Agreement will terminate upon the Closing. | | | ||
| | | | | |||
Right of Co-Sale | | | Ocuphire does not have a right of co-sale in place. | | | Rexahn does not have a right of co-sale in place. |
| | | | | |||
Drag-Along Rights | | | As further described in the Ocuphire Stockholders Agreement, in the event that the Ocuphire Board and holders of a majority of the outstanding capital stock of Ocuphire vote to approve a transaction resulting in a change of control of Ocuphire, the stockholders party to the Ocuphire Stockholders Agreement shall vote all their shares of Ocuphire capital stock in favor of such transaction and sell all their shares of Ocuphire capital stock pursuant to the terms of such transaction. The Ocuphire Stockholders Agreement will terminate immediately prior to the completion of the merger. | | | Rexahn does not have drag along rights in place. |
| | | | | |||
Right of First Offer | | | Ocuphire does not have a right of first offer in place. | | | Rexahn does not have a right of first offer place. |
| | | | | |||
Forum Selection | | | The Ocuphire Certificate of Incorporation and the Ocuphire Bylaws do not provide for a specific forum. | | | The Rexahn Certificate of Incorporation and the Rexahn Bylaws do not provide for a specific forum. |
| | | | | |||
Indemnification of Officers and Directors and Advancement of Expenses; Limitation on Personal Liability | ||||||
| | | | | |||
Indemnification | | | The Ocuphire Certificate of Incorporation and Ocuphire Bylaws provide that Ocuphire shall indemnify its directors and officers to the fullest extent permitted by applicable law. Under the Ocuphire Bylaws, Ocuphire will not be required to indemnify any director or officer in connection with any proceeding initiated by such person unless the proceeding was expressly required by law, authorized by the Ocuphire Board or is provided for by the corporation. Under the Ocuphire Bylaws, such rights shall not be | | | The Rexahn Bylaws provide that Rexahn shall indemnify its directors and officers if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the Rexahn. Under the Rexahn Bylaws, Rexahn will not be required to indemnify any director or officer in connection with any proceeding initiated by such person against Rexahn unless the proceeding was authorized by the Rexahn Board, expressly required by law, or is provided for by the corporation. Under the |
Provision | | | Ocuphire (Pre-Merger) | | | Rexahn (Post-Merger) |
| | | exclusive of any other rights acquired by directors and officers, including by agreement. | | | Rexahn Bylaws, such rights shall not be exclusive of any other rights acquired by directors and officers, including by agreement. | |
| | | | | |||
Advancement of Expenses | | | The Ocuphire Bylaws provide that, to the fullest extent not prohibited by applicable law, Ocuphire will pay the expenses incurred by any director or executive officer in defending any proceeding; provided, however, that, to the extent required by law, such payment of expenses in advance of the final disposition of the proceeding will be made only upon receipt of an undertaking by the person to repay all amounts in advance if it should be ultimately determined that the person is not entitled to be indemnified under the Ocuphire Bylaws or otherwise. | | | The Rexahn Bylaws provide that, to the fullest extent not prohibited by applicable law, Rexahn will pay the expenses incurred by any present or former officer or director in defending any proceeding in advance of its final disposition, provided, however, that, to the extent required by law, such payment of expenses in advance of the final disposition of the proceeding will be made only upon receipt of an undertaking by the person to repay all amounts in advance if it should be ultimately determined that the person is not entitled to be indemnified under the Rexahn Bylaws or otherwise. |
| | | | | |||
Registration Rights | | | The Ocuphire Certificate of Incorporation and Ocuphire Bylaws do not provide registration rights to holders of Ocuphire capital stock. | | | The Rexahn Bylaws and the Rexahn Certificate of Incorporation do not provide registration rights to holders of Rexahn common stock. |
| | | | | |||
Dividends | ||||||
| | | | | |||
Declaration and Payment of Dividends | | | The Ocuphire Bylaws provide that dividends upon Ocuphire capital stock may be declared by the Ocuphire Board at any regular or special meeting. Dividends may be paid in cash, in property, or in shares of the capital stock. The Ocuphire Board may, in its discretion, set aside any company funds available for dividends as a reserve. | | | Holders of Rexahn common stock are entitled to receive dividends as may be lawfully declared from time to time by the Rexahn Board. |
| | | | | |||
Amendments to Certificate of Incorporation or Bylaws | ||||||
| | | | | |||
General Provisions | | | Under the Ocuphire Certificate of Incorporation, Ocuphire reserves the right to amend, alter, change or repeal any provision contained in the Ocuphire Certificate of Incorporation. Ocuphire Stockholders are not entitled to vote on any amendment to the | | | Provisions of the Rexahn Certificate of Incorporation may be amended, altered or repealed in the manner prescribed by the DGCL. The Rexahn Certificate of Incorporation states that the Rexahn Board will have the power to alter, |
Provision | | | Ocuphire (Pre-Merger) | | | Rexahn (Post-Merger) |
| | | Ocuphire Certificate of Incorporation that relates solely to the terms of one or more outstanding series of preferred stock, if the holders of such affected series are entitled to vote thereon by law or pursuant to the Ocuphire Certificate of Incorporation. The Ocuphire Bylaws provide that the Ocuphire Board may adopt, amend or repeal the Ocuphire Bylaws. Any adoption, amendment or repeal of the Ocuphire Bylaws by the Ocuphire Board requires the approval of a majority of the authorized number of directors. Ocuphire Stockholders also have power to adopt, amend or repeal the Ocuphire Bylaws. The affirmative vote of the holders of at least a majority of the voting power of all of the then-outstanding shares of Ocuphire capital stock entitled to vote generally in the election of directors, voting together as a single class, is required to adopt, amend or repeal any provision of the Ocuphire Bylaws. | | | amend or repeal the Rexahn Bylaws in a manner not inconsistent with the DGCL, subject to the power of the holders of common stock to amend or repeal the bylaws made by the Rexahn Board. The Rexahn Bylaws may be amended by the stockholders or by the Rexahn Board, when such power is conferred upon the Rexahn Board by the Rexahn Certificate of Incorporation, at any regular meeting of the stockholders or of the Rexahn Board or at any special meeting of the stockholders or of the Rexahn Board. |
• | each of Rexahn’s directors; |
• | each of Rexahn’s named executive officers; and |
• | all of Rexahn’s current directors and executive officers as a group. |
| | | Shares Beneficially Owned | ||||
Name of Beneficial Owner | | | Number of Shares | | | Percentage |
Directors and Named Executive Officers: | | | | | ||
Douglas J. Swirsky | | | 39,755(1) | | | * |
Lisa Nolan(2) | | | 1,936 | | | * |
Ely Benaim(2) | | | 395 | | | * |
Peter Brandt | | | 13,137(3) | | | * |
Charles Beever | | | 12,637(4) | | | * |
Kwang Soo Cheong | | | 11,746(5) | | | * |
Richard J. Rodgers | | | 10,639(6) | | | * |
Ben Gil Price | | | 7,922(7) | | | * |
Lara Sullivan | | | 5,897(8) | | | * |
All current executive officers and directors as a group (7 persons) | | | 101,733(9) | | | 2.2% |
* | Represents less than 1% of the issued and outstanding shares of our common stock as of September 10, 2020. |
(1) | Includes 35,589 shares of Rexahn common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. |
(2) | Dr. Benaim resigned from Rexahn in March 2019, and Dr. Nolan resigned from Rexahn in September 2019. |
(3) | Includes 11,887 shares of Rexahn common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. |
(4) | Includes 11,721 shares of Rexahn common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. |
(5) | Includes 11,721 shares of Rexahn common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. |
(6) | Includes 10,639 shares of Rexahn common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. |
(7) | Includes 6,672 shares of Rexahn common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. |
(8) | Includes 5,897 shares of Rexahn common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. |
(9) | Includes 94,126 shares of Rexahn common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. |
• | each person, or group of affiliated persons, known by Ocuphire to beneficially own more than 5% of Ocuphire common stock; |
• | each of Ocuphire’s named executive officers; |
• | each of Ocuphire’s directors; and |
• | all of Ocuphire’s directors and executive officers as a group. |
NAME AND ADDRESS OF BENEFICIAL OWNER | | | NUMBER OF SHARES BENEFICIALLY OWNED | | | PERCENT* |
Greater than 5% stockholders | | | | | ||
Apexian Pharmaceuticals, Inc.(1) | | | 738,281 | | | 13.1% |
William Pitlick(2) | | | 232,976 | | | 4.1% |
Altium Growth Fund, L.P.(3) | | | 555,270 | | | 9.9% |
Empery Asset Management LP(4) | | | 555,270 | | | 9.9% |
Directors and Named Executive Officers | | | | | ||
Mina Sooch(5) | | | 987,788 | | | 16.8% |
Alan R. Meyer(6) | | | 507,309 | | | 8.9% |
Bernhard Hoffmann(7) | | | 151,009 | | | 2.7% |
Sean Ainsworth(8) | | | 84,978 | | | 1.5% |
Cam Gallagher(9) | | | 62,207 | | | 1.1% |
James S. Manuso, Ph.D.(10) | | | 58,132 | | | 1.0% |
All current executive officers and directors as a group (6 persons)(11) | | | 1,851,423 | | | 30.2% |
* | The percentage of ownership is based on 5,631,018 shares of Ocuphire common stock outstanding on September 10, 2020, which assumes (i) the automatic conversion of all Ocuphire convertible notes outstanding as of September 10, 2020 (as if the Ocuphire convertible notes were converted pursuant to the terms of the Note Conversion Agreement on September 10, 2020), and (ii) the issuance of Initial Shares in the Pre-Merger Financing (as if such issuance had already occurred). |
(1) | The address for Apexian is 20 North Meridian Street, Suite 801, Indianapolis, IN 46204. With regard to the shares held by Apexian, the members of the board of directors of Apexian (who are: John H. Barnard, David A. Broecker, Homer L. Pearce, Mark R. Kelley, and Martin Haslanger) share voting and investment discretion with respect to these shares. |
(2) | Includes 16,920 shares of Ocuphire common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. The address of William Pitlick is 88 Virginia St. #71, Seattle, WA 98101. |
(3) | Includes 555,270 Initial Shares issuable in the Pre-Merger Financing. Altium Capital Management, LP, the investment manager of Altium Growth Fund, LP, has voting and investment power over these securities. Jacob Gottlieb is the managing member of Altium Capital Growth GP, LLC, which is the general partner of Altium Growth Fund, LP. Each of Altium Growth Fund, LP, Altium Capital Growth GP, LLC and Jacob Gottlieb disclaims beneficial ownership over these shares. The address for Altium Growth Fund, L.P. is c/o Altium Capital Management, LP, 551 5th Avenue, 19th Floor, Suite 1920, New York, NY 10176. |
(4) | Includes (i) 38,869 Initial Shares issuable to Empery Asset Master Ltd (“EAM”), (ii) shares 11,105 Initial Shares issuable to Empery Tax Efficient, LP (“ETE”) and (iii) 505,295 Initial Shares issuable to Empery Debt Opportunity Fund, LP (together with EAM and ETE, the “Empery Entities” and each, an “Empery Entity”). Empery Asset Management LP, the authorized agent of each Empery Entity, has discretionary authority to vote and dispose of the shares held by each Empery Entity and may be deemed to be the beneficial owner of these shares. Martin Hoe and Ryan Lane, in their capacity as investment managers of Empery Asset Management LP, may also be deemed to have investment discretion and voting power over the shares held by the Empery Entities. The Empery Entities, Mr. Hoe and Mr. Lane each disclaim any beneficial ownership of these shares. The address of Empery Asset Management, LP is 1 Rockefeller Plaza, Suite 1205, New York, NY 10020. The address for Empery Asset Management is c/o Empery Asset Management, 1 Rockefeller Plaza, Suite 1205, New York, NY 10020. |
(5) | Includes (i) 241,750 shares of Ocuphire common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020, (ii) 3,609 Initial Shares issuable in the Pre-Merger Financing, and (iii) 22,429 shares issuable upon the automatic conversion of the principal and accrued and unpaid interest outstanding as of September 10, 2020 on Ocuphire’s outstanding convertible notes. |
(6) | Includes (i) 48,356 shares of Ocuphire common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020, (ii) 555 Initial Shares issuable in the Pre-Merger Financing, and (iii) 28,250 shares issuable upon the automatic conversion of the principal and accrued and unpaid interest outstanding as of September 10, 2020 on Ocuphire’s outstanding convertible notes. |
(7) | Includes (i) 56,275 shares of Ocuphire common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020 and (ii) 2,430 shares issuable upon the automatic conversion of the principal and accrued and unpaid interest outstanding as of September 10, 2020 on Ocuphire’s outstanding convertible notes. |
(8) | Includes (i) 51,256 shares of Ocuphire common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020, (ii) 2,776 Initial Shares issuable in the Pre-Merger Financing, and (iii) 10,946 shares issuable upon the automatic conversion of the principal and accrued and unpaid interest outstanding as of September 10, 2020 on Ocuphire’s outstanding convertible notes. |
(9) | Includes (i) 48,656 shares of Ocuphire common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020, (ii) 2,776 Initial Shares issuable in the Pre-Merger Financing, and (iii) 10,775 shares issuable upon the automatic conversion of the principal and accrued and unpaid interest outstanding as of September 10, 2020 on Ocuphire’s outstanding convertible notes. |
(10) | Includes (i) 48,656 shares of Ocuphire common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020, (ii) 1,388 Initial Shares issuable in the Pre-Merger Financing, and (iii) 8,088 shares issuable upon the automatic conversion of the principal and accrued and unpaid interest outstanding as of September 10, 2020 on Ocuphire’s outstanding convertible notes. |
(11) | Includes (i) 494,949 shares of Ocuphire common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020, (ii) 11,104 Initial Shares issuable in the Pre-Merger Financing, and (iii) 82,918 shares issuable upon the automatic conversion of the principal and accrued and unpaid interest outstanding as of September 10, 2020 on Ocuphire’s outstanding convertible notes. |
• | each person, or group of affiliated persons, expected by Ocuphire and Rexahn to become the beneficial owner of more than 5% of the common stock of the combined company upon the consummation of the merger; |
• | each named executive officer of the combined company; |
• | each director of the combined company; and |
• | all of the combined company’s directors and executive officers as a group. |
| | | Beneficial Ownership | ||||
Name of Beneficial Owner | | | Shares | | | % |
Greater than 5% Stockholders: | | | | | ||
Apexian Pharmaceuticals, Inc.(1) | | | 3,234,556 | | | 11.1% |
Altium Growth Fund, L.P.(2) | | | 2,432,748 | | | 8.3% |
Empery Asset Management LP(3) | | | 2,432,744 | | | 8.3% |
Current Executive Officers and Directors: | | | | | ||
Mina Sooch(4) | | | 4,327,696 | | | 14.3% |
Bernhard Hoffmann(5) | | | 661,600 | | | 2.3% |
Sean Ainsworth(6) | | | 372,304 | | | 1.3% |
James S. Manuso(7) | | | 254,687 | | | * |
Cam Gallagher(8) | | | 272,540 | | | * |
Alan R. Meyer(9) | | | 2,222,621 | | | 7.6% |
Richard J. Rodgers | | | 24,328 | | | * |
Susan Benton | | | — | | | 0 |
All current executive officers and directors as a group (8 persons)(10) | | | 8,135,776 | | | 26.0% |
(1) | The address for Apexian is 20 North Meridian Street, Suite 801, Indianapolis, IN 46204. With regard to the shares held by Apexian, the members of the board of directors of Apexian (who are: John H. Barnard, David A. Broecker, Homer L. Pearce, Mark R. Kelley, and Martin Haslanger) share voting and investment discretion with respect to these shares. |
(2) | Altium Capital Management, LP, the investment manager of Altium Growth Fund, LP, has voting and investment power over these securities. Jacob Gottlieb is the managing member of Altium Capital Growth GP, LLC, which is the general partner of Altium Growth Fund, LP. Each of Altium Growth Fund, LP, Altium Capital Growth GP, LLC and Jacob Gottlieb disclaims beneficial ownership over these shares. The address for Altium Growth Fund, L.P. is c/o Altium Capital Management, LP, 551 5th Avenue, 19th Floor, Suite 1920, New York, NY 10176. |
(3) | Includes (i) 186,617 shares of common stock held by Empery Asset Master Ltd (“EAM”), (ii) 57,734 shares of common stock held by Empery Tax Efficient, LP (“ETE”) and (iii) 2,244,572 shares of common stock held by Empery Debt Opportunity Fund, LP (together with EAM and ETE, the “Empery Entities” and each, an “Empery Entity”). Empery Asset Management LP, the authorized agent of each Empery Entity, has discretionary authority to vote and dispose of the shares held by each Empery Entity and may be deemed to be the beneficial owner of these shares. Martin Hoe and Ryan Lane, in their capacity as investment managers of Empery Asset Management LP, may also be deemed to have investment discretion and voting power over the shares held by the Empery Entities. The Empery Entities, Mr. Hoe and Mr. Lane each disclaim any beneficial ownership of these shares. The address of Empery Asset Management, LP is 1 Rockefeller Plaza, Suite 1205, New York, NY 10020. The address for Empery Asset Management is c/o Empery Asset Management, 1 Rockefeller Plaza, Suite 1205, New York, NY 10020. |
(4) | Includes 1,059,155 shares of combined company common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. |
(5) | Includes 246,552 shares of combined company common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. |
(6) | Includes 224,562 shares of combined company common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. |
(7) | Includes 213,171 shares of combined company common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. |
(8) | Includes 213,171 shares of combined company common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. |
(9) | Includes 211,857 shares of combined company common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. |
(10) | Includes 2,168,468 shares of combined company common stock underlying options to purchase common stock that are exercisable within 60 days of September 10, 2020. |
Rexahn Pharmaceuticals, Inc. 15245 Shady Grove Road, Suite 455 Rockville, Maryland 20850 Telephone: (240) 268-5300 Attn: Secretary | | | Ocuphire Pharma, Inc. 37000 Grand River Avenue, Suite 120 Farmington Hills, MI 48335 Telephone: (248) 681-9815 Attn: Secretary |
| | | Page | |
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | |
| | | Page | |
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | | ||
| | |
| | | December 31, 2019 | | | December 31, 2018 | |
ASSETS | | | | | ||
Current Assets: | | | | | ||
Cash and cash equivalents | | | $9,219,547 | | | $8,744,301 |
Marketable securities | | | 2,997,220 | | | 5,981,520 |
Prepaid expenses and other current assets | | | 447,206 | | | 1,173,847 |
Total Current Assets | | | 12,663,973 | | | 15,899,668 |
Security Deposits | | | 25,681 | | | 30,785 |
Operating Lease Right-of-Use Assets | | | 203,348 | | | — |
Equipment, Net | | | 75,770 | | | 112,473 |
Total Assets | | | $12,968,772 | | | $16,042,926 |
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | ||
Current Liabilities: | | | | | ||
Accounts payable and accrued expenses | | | $1,265,731 | | | $3,152,550 |
Deferred revenue | | | 1,500,000 | | | — |
Operating lease liabilities, current | | | 139,765 | | | — |
Total Current Liabilities | | | 2,905,496 | | | 3,152,550 |
Operating Lease Liabilities, non-current | | | 63,605 | | | — |
Warrant Liabilities | | | 41,717 | | | 2,307,586 |
Other Liabilities | | | — | | | 19,900 |
Total Liabilities | | | 3,010,818 | | | 5,480,036 |
Commitments and Contingencies (note 14) | | | | | ||
Stockholders’ Equity: | | | | | ||
Preferred stock, par value $0.0001, 10,000,000 authorized shares, none issued and outstanding | | | — | | | — |
Common stock, par value $0.0001, 75,000,000 authorized shares, 4,019,141 and 3,122,843 issued and outstanding | | | 402 | | | 312 |
Additional paid-in capital | | | 173,278,144 | | | 165,267,656 |
Accumulated other comprehensive income (loss) | | | 2,084 | | | (17,836) |
Accumulated deficit | | | (163,322,676) | | | (154,687,242) |
Total Stockholders’ Equity | | | 9,957,954 | | | 10,562,890 |
Total Liabilities and Stockholders’ Equity | | | $12,968,772 | | | $16,042,926 |
| | | For the Years Ended December 31, | ||||
| | | 2019 | | | 2018 | |
Revenues: | | | $— | | | $— |
Expenses: | | | | | ||
General and administrative | | | 5,738,227 | | | 7,428,615 |
Research and development | | | 5,476,776 | | | 13,109,058 |
Total Expenses | | | 11,215,003 | | | 20,537,673 |
Loss from Operations | | | (11,215,003) | | | (20,537,673) |
Other Income | | | | | ||
Interest income | | | 313,700 | | | 254,344 |
Other income | | | — | | | 368,750 |
Unrealized gain on fair value of warrants | | | 2,265,869 | | | 5,546,049 |
Total Other Income | | | 2,579,569 | | | 6,169,143 |
Net Loss Before Provision for Income Taxes | | | (8,635,434) | | | (14,368,530) |
Provision for income taxes | | | — | | | — |
Net Loss | | | $(8,635,434) | | | $(14,368,530) |
Net loss per share, basic and diluted | | | $(2.18) | | | $(5.25) |
Weighted average number of shares outstanding, basic and diluted | | | 3,960,163 | | | 2,738,506 |
| | | Common Stock | | | Additional Paid-in Capital | | | Accumulated Deficit | | | Accumulated Other Comprehensive Income (Loss) | | | Total Stockholders’ Equity | ||||
| | | Number of Shares | | | Amount | | ||||||||||||
Balances at January 1, 2018 | | | 2,639,319 | | | $264 | | | $157,143,930 | | | $(140,318,712) | | | $(56,886) | | | $16,768,596 |
Issuance of common stock and units, net of issuance costs | | | 480,770 | | | 48 | | | 6,872,741 | | | — | | | — | | | 6,872,789 |
Common stock issued in exchange for services | | | 1,250 | | | — | | | 22,650 | | | — | | | — | | | 22,650 |
Common stock issued from vested restricted stock units | | | 1,504 | | | — | | | — | | | — | | | — | | | — |
Stock-based compensation | | | — | | | — | | | 1,228,335 | | | — | | | — | | | 1,228,335 |
Net loss | | | — | | | — | | | — | | | (14,368,530) | | | — | | | (14,368,530) |
Other comprehensive income | | | — | | | — | | | — | | | — | | | 39,050 | | | 39,050 |
Balances at December 31, 2018 | | | 3,122,843 | | | $312 | | | $165,267,656 | | | $(154,687,242) | | | $(17,836) | | | $10,562,890 |
Issuance of common stock and units, net of issuance costs | | | 895,834 | | | 90 | | | 7,553,738 | | | — | | | — | | | 7,553,828 |
Common stock issued from vested restricted stock units | | | 464 | | | — | | | — | | | — | | | — | | | — |
Stock-based compensation | | | — | | | — | | | 456,750 | | | — | | | — | | | 456,750 |
Net loss | | | — | | | — | | | — | | | (8,635,434) | | | — | | | (8,635,434) |
Other comprehensive income | | | — | | | — | | | — | | | — | | | 19,920 | | | 19,920 |
Balances at December 31, 2019 | | | 4,019,141 | | | $402 | | | $173,278,144 | | | $(163,322,676) | | | $2,084 | | | $9,957,954 |
| | | For the Year Ended December 31, | ||||
| | | 2019 | | | 2018 | |
Cash Flows from Operating Activities: | | | | | ||
Net loss | | | $(8,635,434) | | | $(14,368,530) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | ||
Compensatory stock | | | — | | | 22,650 |
Depreciation and amortization | | | 40,992 | | | 48,211 |
Loss on sale of equipment | | | 9,594 | | | — |
Amortization of premiums and discounts on marketable securities, net | | | (108,214) | | | 39,251 |
Stock-based compensation | | | 456,750 | | | 1,228,335 |
Amortization and termination of deferred research and development arrangement | | | — | | | (375,000) |
Unrealized gain on fair value of warrants | | | (2,265,869) | | | (5,546,049) |
Changes in assets and liabilities: | | | | | ||
Prepaid expenses and other assets | | | 608,143 | | | 230,694 |
Accounts payable and accrued expenses | | | (1,886,819) | | | (81,376) |
Deferred revenue | | | 1,500,000 | | | — |
Other, net | | | 3,724 | | | (36,824) |
Net Cash Used in Operating Activities | | | (10,277,133) | | | (18,838,638) |
Cash Flows from Investing Activities: | | | | | ||
Purchase of equipment | | | (19,383) | | | (39,224) |
Sale of equipment | | | 5,500 | | | — |
Purchase of marketable securities | | | (8,887,566) | | | — |
Redemption of marketable securities | | | 12,000,000 | | | 11,950,220 |
Net Cash Provided by Investing Activities | | | 3,098,551 | | | 11,910,996 |
Cash Flows from Financing Activities: | | | | | ||
Issuance of common stock and units, net of issuance costs | | | 7,653,828 | | | 6,872,789 |
Payment of deferred offering costs | | | — | | | (100,000) |
Net Cash Provided by Financing Activities | | | 7,653,828 | | | 6,772,789 |
Net Increase (Decrease) in Cash and Cash Equivalents | | | 475,246 | | | (154,853) |
Cash and Cash Equivalents – beginning of period | | | 8,744,301 | | | 8,899,154 |
Cash and Cash Equivalents – end of period | | | $9,219,547 | | | $8,744,301 |
Supplemental Cash Flow Information | | | | | ||
Operating cash flows paid for amounts included in the measurement of lease liabilities | | | $197,224 | | | $— |
Non-cash financing and investing activities: | | | | | ||
Warrants issued | | | $4,735,913 | | | $4,841,830 |
Operating lease right-of-use assets obtained in exchange for lease obligations: | | | $380,935 | | | $— |
1. | Operations and Organization |
2. | Summary of Significant Accounting Policies |
| | | Life | | | Depreciation Method | |
Furniture and fixtures | | | 7 years | | | straight line |
Office equipment | | | 5 years | | | straight line |
Laboratory equipment | | | 5-7 years | | | straight line |
Computer equipment | | | 3-5 years | | | straight line |
Leasehold improvements | | | 3-5 years | | | straight line |
3. | Marketable Securities |
| | | December 31, 2019 | ||||||||||
| | | Cost Basis | | | Gross Unrealized Gains | | | Gross Unrealized Losses | | | Fair Value | |
Commercial Paper | | | $1,996,216 | | | $1,184 | | | $ — | | | $1,997,400 |
Corporate Bonds | | | 998,920 | | | 900 | | | — | | | 999,820 |
Total Marketable Securities | | | $2,995,136 | | | $2,084 | | | $— | | | $2,997,220 |
| | | December 31, 2018 | ||||||||||
| | | Cost Basis | | | Gross Unrealized Gains | | | Gross Unrealized Losses | | | Fair Value | |
Corporate Bonds | | | $5,999,356 | | | $ — | | | $(17,836) | | | $5,981,520 |
4. | Prepaid Expenses and Other Current Assets |
| | | December 31, 2019 | | | December 31, 2018 | |
Deposits on contracts | | | $— | | | $618,417 |
Prepaid expenses and other current assets | | | 447,206 | | | 555,430 |
| | | $447,206 | | | $1,173,847 |
5. | Equipment, Net |
| | | December 31, 2019 | | | December 31, 2018 | |
Furniture and fixtures | | | $67,650 | | | $82,686 |
Office and computer equipment | | | 163,440 | | | 159,489 |
Laboratory equipment | | | — | | | 447,653 |
Leasehold improvements | | | 116,403 | | | 131,762 |
Total equipment | | | 347,493 | | | 821,590 |
Less: Accumulated depreciation and amortization | | | (271,723) | | | (709,117) |
Net carrying amount | | | $75,770 | | | $112,473 |
6. | Accounts Payable and Accrued Expenses |
| | | December 31, 2019 | | | December 31, 2018 | |
Trade payables | | | $488,285 | | | $547,519 |
Accrued expenses | | | 471,700 | | | 140,637 |
Accrued research and development contract costs | | | 221,170 | | | 1,782,131 |
Payroll liabilities | | | 84,576 | | | 682,263 |
| | | $1,265,731 | | | $3,152,550 |
7. | Collaboration and License Agreements |
8. | Leases |
Right-of-Use Assets | | | $203,348 |
Operating Lease Liabilities | | | |
Current | | | $139,765 |
Long Term | | | 63,605 |
Total Operating Lease Liabilities | | | $203,370 |
Year Ending December 31: | | | |
2020 | | | $155,280 |
2021 | | | 65,364 |
Minimum lease payments | | | 220,644 |
Less: Imputed interest | | | (17,274) |
Present value of minimum lease payments | | | 203,370 |
Less: current maturities of lease obligations | | | (139,765) |
Long-term lease obligations | | | $63,605 |
9. | Net Loss per Common Share |
10. | Common Stock |
11. | Stock-Based Compensation |
| | | For the Year Ended December 31, | ||||
| | | 2019 | | | 2018 | |
Statement of operations line item: | | | | | ||
General and administrative | | | $393,483 | | | $883,855 |
Research and development | | | 63,267 | | | 344,480 |
Total | | | $456,750 | | | $1,228,335 |
| | | For the Year Ended December 31, | ||||
| | | 2019 | | | 2018 | |
Black-Scholes assumptions | | | | | ||
Expected dividend yield | | | 0% | | | 0% |
Expected volatility | | | 74-75% | | | 69-73% |
Risk-free interest rate | | | 1.9-2.6% | | | 2.3-2.9% |
Expected term (in years) | | | 5.5-6 years | | | 5.5-6 years |
| | | Number of Options | | | Weighted Average Exercise Price | | | Weighted Average Remaining Contractual Term | | | Aggregate Intrinsic Value | |
Outstanding, January 1, 2019 | | | 255,922 | | | $41.88 | | | 7.8 years | | | $ — |
Granted | | | 52,465 | | | $6.41 | | | | | ||
Exercised | | | — | | | $— | | | | | ||
Expired | | | (2,080) | | | $97.78 | | | | | ||
Cancelled | | | (101,733) | | | $34.99 | | | | | ||
Outstanding, December 31, 2019 | | | 204,574 | | | $35.60 | | | 7.3 years | | | $— |
Exercisable, December 31, 2019 | | | 123,263 | | | $51.15 | | | 6.2 years | | | $— |
| | | 2019 | ||||
| | | Number of Options | | | Weighted Average Fair Value at Grant Date | |
Unvested at January 1, 2019 | | | 131,531 | | | $13.19 |
Granted | | | 52,465 | | | $4.20 |
Vested | | | (51,386) | | | $14.12 |
Cancelled | | | (51,299) | | | $11.47 |
Unvested at December 31, 2019 | | | 81,311 | | | $7.90 |
| | | Number of RSUs | | | Weighted Average Grant Date Fair Value | |
Outstanding, January 1, 2019 | | | 1,394 | | | $22.08 |
Granted | | | — | | | $— |
Vested and Released | | | (464) | | | $22.08 |
Cancelled | | | (930) | | | $22.08 |
Outstanding, December 31, 2019 | | | — | | | $— |
12. | Warrants |
| | | Number of Warrants: | | | | | ||||||
Warrant Issuance | | | December 31, 2019 | | | December 31, 2018 | | | Exercise Price | | | Expiration Date |
Liability-classified Warrants | | | | | | | | | ||||
January 2014 Investors | | | — | | | 39,683 | | | $153.60 | | | Jan. 2019 |
November 2015 Investors | | | 104,168 | | | 104,168 | | | $63.60 | | | May 2021 |
November 2015 Placement Agent | | | 279 | | | 279 | | | $63.60 | | | Nov. 2020 |
March 2016 Investors | | | 50,651 | | | 50,651 | | | $50.40 | | | Sept.2021 |
September 2016 Investors | | | 67,084 | | | 67,084 | | | $36.00 | | | Mar. 2022 |
June 2017 Investors | | | 126,264 | | | 126,264 | | | $48.00 | | | Dec. 2022 |
June 2017 Placement Agent | | | 15,153 | | | 15,153 | | | $49.50 | | | June 2022 |
October 2017 Investors | | | 136,058 | | | 136,058 | | | $34.20 | | | Apr. 2023 |
October 2017 Placement Agent | | | 16,327 | | | 16,327 | | | $36.72 | | | Oct. 2022 |
Total liability classified warrants | | | 515,984 | | | 555,667 | | | | | ||
| | | | | | | | | |||||
Equity-classified Warrants | | | | | | | | | ||||
October 2018 Investors | | | 480,771 | | | 480,771 | | | $20.04 | | | Apr. 2024 |
October 2018 Placement Agent | | | 28,848 | | | 28,848 | | | $19.50 | | | Oct. 2023 |
January 2019 Investors | | | 895,886 | | | — | | | $9.60 | | | Jan. 2024 |
Total equity-classified warrants | | | 1,405,505 | | | 509,619 | | | | | ||
Total outstanding warrants | | | 1,921,489 | | | 1,065,286 | | | | | ||
| | | Number of Warrants | | | Total | | | Weighted average exercise price | ||||
| | | Liability- classified | | | Equity- classified | | ||||||
Balance, January 1, 2019 | | | 555,667 | | | 509,619 | | | 1,065,286 | | | $37.52 |
Issued during the period | | | — | | | 895,886 | | | 895,886 | | | $9.60 |
Exercised during the period | | | — | | | — | | | — | | | $— |
Expired during the period | | | (39,683) | | | — | | | (39,683) | | | $153.60 |
Balance, December 31, 2019 | | | 515,984 | | | 1,405,505 | | | 1,921,489 | | | $22.10 |
| | | Fair Value as of: | ||||
Warrant Issuance: | | | December 31, 2019 | | | December 31, 2018 |
November 2015 Investors | | | $55 | | | $234,918 |
November 2015 Placement Agent | | | — | | | 435 |
March 2016 Investor | | | 439 | | | 160,099 |
September 2016 Investors | | | 3,196 | | | 333,834 |
June 2017 Investors | | | 11,736 | | | 623,324 |
June 2017 Placement Agent | | | 845 | | | 65,149 |
October 2017 Investors | | | 23,772 | | | 801,551 |
October 2017 Placement Agent | | | 1,674 | | | 88,276 |
Total: | | | $41,717 | | | $2,307,586 |
| | | December 31, 2019 | | | December 31, 2018 | |
Trading market prices | | | $1.91 | | | $11.16 |
Estimated future volatility | | | 102% | | | 105% |
Dividend | | | — | | | — |
Estimated future risk-free rate | | | 1.57-1.72% | | | 2.35-2.53% |
Equivalent volatility | | | 85-94% | | | 99-104% |
Equivalent risk-free rate | | | 1.57-1.59% | | | 2.51-2.55% |
Fundamental transaction likelihood | | | 50% | | | 5% |
Fundamental transaction timing | | | April 2020 | | | End of warrant term |
| | | For the Year Ended December 31, | ||||
| | | 2019 | | | 2018 | |
Expired Warrants | | | $— | | | $64,307 |
November 2015 Investors | | | 234,863 | | | 1,025,132 |
November 2015 Placement Agent | | | 435 | | | 2,501 |
March 2016 Investors | | | 159,660 | | | 537,455 |
September 2016 Investors | | | 330,638 | | | 720,249 |
June 2017 Investors | | | 611,588 | | | 1,358,540 |
June 2017 Placement Agent | | | 64,304 | | | 156,442 |
October 2017 Investors | | | 777,779 | | | 1,504,001 |
October 2017 Placement Agent | | | 86,602 | | | 177,422 |
Total: | | | $2,265,869 | | | $5,546,049 |
13. | Income Taxes |
| | | December 31, 2019 | | | December 31, 2018 | |
Net Operating Loss Carryforwards | | | $43,844,000 | | | $41,184,000 |
Stock Compensation Expense | | | 1,191,000 | | | 1,608,000 |
Book Tax Differences on Assets and Liabilities | | | 464,000 | | | 195,000 |
Valuation Allowance | | | (45,499,000) | | | (42,987,000) |
Net Deferred Tax Assets | | | $— | | | $— |
14. | Commitments and Contingencies |
a) | The Company has contracted with various vendors for research and development services, with terms that require payments over the term of the agreements, usually ranging from two to 36 months. The costs to be incurred are estimated and are subject to revision. As of December 31, 2019, the total estimated cost to complete these agreements was approximately $1,750,000. All of these agreements may be terminated by either party upon appropriate notice as stipulated in the respective agreements. |
b) | On June 22, 2009, the Company entered into a License Agreement with Korea Research Institute of Chemical Technology (“KRICT”) to acquire the rights to all intellectual property related to quinoxaline-piperazine derivatives that were synthesized under a Joint Research Agreement. The agreement with KRICT calls for a one-time milestone payment of $1,000,000 within 30 days after the first achievement of marketing approval of the first commercial product arising out of or in connection with the use of KRICT’s intellectual property. As of December 31, 2019, the milestone has not occurred. |
c) | The Company has established a 401(k) plan for its employees. The Company has elected to match 100% of the first 3% of an employee’s compensation plus 50% of an additional 2% of the employee’s deferral. Expense related to this matching contribution aggregated to $71,568 and $120,558, for the years ended December 31, 2019 and 2018 respectively. |
d) | On February 5, 2018, the Company and Next BT terminated the research collaboration agreement between the Company and Rexgene. In exchange for Next BT terminating its rights to RX-0201 in Asia, the Company agreed to pay Next BT a royalty in the low single digits of any net sales of RX-0201 the Company makes in Asia and 50% of the Company’s licensing revenue related to licensing of RX-0201 in Asia, up to an aggregate of $5,000,000. As of December 31, 2019, the Company has not made any royalty payments to Next BT. |
15. | Fair Value Measurements |
Level 1 Inputs — | Unadjusted quoted prices in active markets for identical assets or liabilities that are accessible by the Company; |
Level 2 Inputs — | Quoted prices in markets that are not active or financial instruments for which all significant inputs are observable, either directly or indirectly; |
Level 3 Inputs — | Unobservable inputs for the asset or liability including significant assumptions of the Company and other market participants. |
Fair Value Measurements at December 31, 2019 | ||||||||||||
| | | Total | | | Level 1 | | | Level 2 | | | Level 3 | |
Assets: | | | | | | | | | ||||
Commercial Paper | | | $1,997,400 | | | $ — | | | $1,997,400 | | | $— |
Corporate Bonds | | | 999,820 | | | — | | | 999,820 | | | — |
Total Assets: | | | $2,997,220 | | | $— | | | $2,997,220 | | | $— |
Liabilities: | | | | | | | | | ||||
Warrant Liabilities | | | $41,717 | | | $— | | | $— | | | $41,717 |
Fair Value Measurements at December 31, 2018 | ||||||||||||
| | | Total | | | Level 1 | | | Level 2 | | | Level 3 | |
Assets: | | | | | | | | | ||||
Corporate Bonds | | | $5,981,520 | | | $ — | | | $5,981,520 | | | $— |
Liabilities: | | | | | | | | | ||||
Warrant Liabilities | | | $2,307,586 | | | $— | | | $— | | | $2,307,586 |
| | | Warrant Liabilities | |
Balance at January 1, 2019 | | | $2,307,586 |
Unrealized gains, net | | | (2,265,869) |
Balance at December 31, 2019 | | | $41,717 |
| | | Warrant Liabilities | |
Balance at January 1, 2018 | | | $7,853,635 |
Unrealized gains, net | | | (5,546,049) |
Balance at December 31, 2018 | | | $2,307,586 |
16. | Subsequent Event |
| | | June 30, 2020 | | | December 31, 2019 | |
ASSETS | ||||||
Current Assets: | | | | | ||
Cash and cash equivalents | | | $9,208,951 | | | $9,219,547 |
Marketable securities | | | — | | | 2,997,220 |
Prepaid expenses and other current assets | | | 817,653 | | | 447,206 |
Total Current Assets | | | 10,026,604 | | | 12,663,973 |
Security Deposits | | | 25,681 | | | 25,681 |
Operating Lease Right-of-Use Assets | | | 139,477 | | | 203,348 |
Equipment, Net | | | 57,312 | | | 75,770 |
Total Assets | | | $10,249,074 | | | $12,968,772 |
LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||
Current Liabilities: | | | | | ||
Accounts payable and accrued expenses | | | $2,190,753 | | | $1,265,731 |
Deferred revenue | | | 650,000 | | | 1,500,000 |
Operating lease liabilities, current | | | 136,197 | | | 139,765 |
Total Current Liabilities | | | 2,976,950 | | | 2,905,496 |
Operating Lease Liabilities, non-current | | | — | | | 63,605 |
Warrant Liabilities | | | 268,811 | | | 41,717 |
Total Liabilities | | | 3,245,761 | | | 3,010,818 |
Commitments and Contingencies (Note 12) | | | | | ||
Stockholders’ Equity: | | | | | ||
Preferred stock, par value $0.0001, 10,000,000 authorized shares, none issued and outstanding | | | — | | | — |
Common stock, par value $0.0001, 75,000,000 authorized shares, 4,019,141 issued and outstanding | | | 402 | | | 402 |
Additional paid-in capital | | | 173,423,515 | | | 173,278,144 |
Accumulated other comprehensive income | | | — | | | 2,084 |
Accumulated deficit | | | (166,420,604) | | | (163,322,676) |
Total Stockholders’ Equity | | | 7,003,313 | | | 9,957,954 |
Total Liabilities and Stockholders’ Equity | | | $10,249,074 | | | $12,968,772 |
| | | For the Three Months Ended June 30, | | | For the Six Months Ended June 30, | |||||||
| | | 2020 | | | 2019 | | | 2020 | | | 2019 | |
Revenues | | | $— | | | $— | | | $1,150,000 | | | $— |
Expenses: | | | | | | | | | ||||
General and administrative | | | 2,116,891 | | | 1,340,016 | | | 3,372,898 | | | 3,035,538 |
Research and development | | | 231,607 | | | 1,648,401 | | | 688,397 | | | 3,890,631 |
Total Expenses | | | 2,348,498 | | | 2,988,417 | | | 4,061,295 | | | 6,926,169 |
Loss from Operations | | | (2,348,498) | | | (2,988,417) | | | (2,911,295) | | | (6,926,169) |
Other Income | | | | | | | | | ||||
Interest income | | | 6,042 | | | 96,650 | | | 40,461 | | | 178,035 |
Unrealized (loss) gain on fair value of warrants | | | (168,702) | | | 427,483 | | | (227,094) | | | 1,940,854 |
Total Other (Loss) Income | | | (162,660) | | | 524,133 | | | (186,633) | | | 2,118,889 |
Net Loss Before Provision for Income Taxes | | | (2,511,158) | | | (2,464,284) | | | (3,097,928) | | | (4,807,280) |
Provision for Income Taxes | | | — | | | — | | | — | | | — |
Net Loss | | | $(2,511,158) | | | $(2,464,284) | | | $(3,097,928) | | | $(4,807,280) |
Net loss per share, basic and diluted | | | $(0.62) | | | $(0.61) | | | $(0.77) | | | $(1.23) |
Weighted average number of shares outstanding, basic and diluted | | | 4,019,141 | | | 4,019,141 | | | 4,019,141 | | | 3,900,208 |
| | | For the Three Months Ended June 30, | | | For the Six Months Ended June 30, | |||||||
| | | 2020 | | | 2019 | | | 2020 | | | 2019 | |
Net Loss | | | $(2,511,158) | | | $(2,464,284) | | | $(3,097,928) | | | $(4,807,280) |
Unrealized gain (loss) on available-for-sale securities | | | — | | | 19,781 | | | (2,084) | | | 25,015 |
Comprehensive Loss | | | $(2,511,158) | | | $(2,444,503) | | | $(3,100,012) | | | $(4,782,265) |
| | | Common Stock | | | Additional Paid-in Capital | | | Accumulated Deficit | | | Accumulated Other Comprehensive Income (Loss) | | | Total Stockholders' Equity | ||||
| | | Number of Shares | | | Amount | | ||||||||||||
Balances at April 1, 2020 | | | 4,019,141 | | | $402 | | | $173,354,446 | | | $(163,909,446) | | | $— | | | $9,445,402 |
Stock-based compensation | | | — | | | — | | | 69,069 | | | — | | | — | | | 69,069 |
Net loss | | | — | | | — | | | — | | | (2,511,158) | | | — | | | (2,511,158) |
Balances at June 30, 2020 | | | 4,019,141 | | | $402 | | | $173,423,515 | | | $(166,420,604) | | | $— | | | $7,003,313 |
| | | | | | | | | | | | | |||||||
Balances at April 1, 2019 | | | 4,019,141 | | | $402 | | | $172,982,394 | | | $(157,030,238) | | | $(12,602) | | | $15,939,956 |
Stock-based compensation | | | — | | | — | | | 127,653 | | | — | | | — | | | 127,653 |
Net loss | | | — | | | — | | | — | | | (2,464,284) | | | — | | | (2,464,284) |
Other comprehensive income | | | — | | | — | | | — | | | — | | | 19,781 | | | 19,781 |
Balances at June 30, 2019 | | | 4,019,141 | | | $402 | | | $173,110,047 | | | $(159,494,522) | | | $7,179 | | | $13,623,106 |
| | | | | | | | | | | | | |||||||
Balances at January 1, 2020 | | | 4,019,141 | | | $402 | | | $173,278,144 | | | $(163,322,676) | | | $2,084 | | | $9,957,954 |
Stock-based compensation | | | — | | | — | | | 145,371 | | | — | | | — | | | 145,371 |
Net loss | | | — | | | — | | | — | | | (3,097,928) | | | — | | | (3,097,928) |
Other comprehensive loss | | | — | | | — | | | — | | | — | | | (2,084) | | | (2,084) |
Balances at June 30, 2020 | | | 4,019,141 | | | $402 | | | $173,423,515 | | | $(166,420,604) | | | $— | | | $7,003,313 |
| | | | | | | | | | | | | |||||||
Balances at January 1, 2019 | | | 3,122,843 | | | $312 | | | $165,267,656 | | | $(154,687,242) | | | $(17,836) | | | $10,562,890 |
Issuance of common stock and units, net of issuance costs | | | 895,834 | | | 90 | | | 7,553,738 | | | — | | | — | | | 7,553,828 |
Common stock issued from vested restricted stock units | | | 464 | | | — | | | — | | | — | | | — | | | — |
Stock-based compensation | | | — | | | — | | | 288,653 | | | — | | | — | | | 288,653 |
Net loss | | | — | | | — | | | — | | | (4,807,280) | | | — | | | (4,807,280) |
Other comprehensive income | | | — | | | — | | | — | | | — | | | 25,015 | | | 25,015 |
Balances at June 30, 2019 | | | 4,019,141 | | | $402 | | | $173,110,047 | | | $(159,494,522) | | | $7,179 | | | $13,623,106 |
| | | For the Six Months Ended June 30, | ||||
| | | 2020 | | | 2019 | |
Cash Flows from Operating Activities: | | | | | ||
Net loss | | | $(3,097,928) | | | $(4,807,280) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | ||
Depreciation and amortization | | | 18,458 | | | 22,077 |
Loss on sale of equipment | | | — | | | 9,594 |
Amortization of premiums and discounts on marketable securities, net | | | (4,864) | | | (56,019) |
Stock-based compensation | | | 145,371 | | | 288,653 |
Unrealized loss (gain) on fair value of warrants | | | 227,094 | | | (1,940,854) |
Changes in assets and liabilities: | | | | | ||
Prepaid expenses and other assets | | | (370,447) | | | 22,308 |
Accounts payable and accrued expenses | | | 925,022 | | | (1,231,816) |
Deferred revenue | | | (850,000) | | | 1,500,000 |
Other, net | | | (3,302) | | | 6,706 |
Net Cash Used in Operating Activities | | | (3,010,596) | | | (6,186,631) |
Cash Flows from Investing Activities: | | | | | ||
Purchase of equipment | | | — | | | (19,383) |
Sale of equipment | | | — | | | 5,500 |
Purchase of marketable securities | | | — | | | (8,887,566) |
Redemption of marketable securities | | | 3,000,000 | | | 6,000,000 |
Net Cash Provided by (Used in) Investing Activities | | | 3,000,000 | | | (2,901,449) |
Cash Flows from Financing Activities: | | | | | ||
Issuance of common stock and units, net of issuance costs | | | — | | | 7,653,828 |
Net Cash Provided by Financing Activities | | | — | | | 7,653,828 |
Net Decrease in Cash and Cash Equivalents | | | (10,596) | | | (1,434,252) |
Cash and Cash Equivalents - Beginning of Period | | | 9,219,547 | | | 8,744,301 |
Cash and Cash Equivalents - End of Period | | | $9,208,951 | | | $7,310,049 |
Supplemental Cash Flow Information | | | | | ||
Operating cash flows paid for amounts included in the measurement of lease liabilities | | | $76,843 | | | $120,700 |
Non-cash financing and investing activities: | | | | | ||
Warrants issued | | | $— | | | $4,735,913 |
Operating lease right-of-use assets obtained in exchange for lease obligations | | | $— | | | $380,935 |
Operations and Organization |
2. | Merger Agreement and Pre-Merger Financing |
3. | Marketable Securities |
| | | December 31, 2019 | ||||||||||
| | | Cost Basis | | | Gross Unrealized Gains | | | Gross Unrealized Losses | | | Fair Value | |
Commercial Paper | | | $1,996,216 | | | $1,184 | | | $— | | | $1,997,400 |
Corporate Bonds | | | 998,920 | | | 900 | | | — | | | 999,820 |
Total Marketable Securities | | | $2,995,136 | | | $2,084 | | | $— | | | $2,997,220 |
4. | Equipment, Net |
| | | June 30, 2020 | | | December 31, 2019 | |
Furniture and fixtures | | | $67,650 | | | $67,650 |
Office and computer equipment | | | 163,440 | | | 163,440 |
Leasehold improvements | | | 116,403 | | | 116,403 |
Total equipment | | | 347,493 | | | 347,493 |
Less: Accumulated depreciation and amortization | | | (290,181) | | | (271,723) |
Net carrying amount | | | $57,312 | | | $75,770 |
5. | Accounts Payable and Accrued Expenses |
| | | June 30, 2020 | | | December 31, 2019 | |
Trade payables | | | $1,651,659 | | | $488,285 |
Accrued expenses | | | 454,850 | | | 471,700 |
Accrued research and development contract costs | | | — | | | 221,170 |
Payroll liabilities | | | 84,244 | | | 84,576 |
| | | $2,190,753 | | | $1,265,731 |
6. | License Agreements |
7. | Leases |
| | | For the Three Months Ended June 30, | | | For the Six Months Ended June 30, | |||||||
| | | 2020 | | | 2019 | | | 2020 | | | 2019 | |
Operating lease cost | | | $36,770 | | | $47,129 | | | $73,541 | | | $127,407 |
Variable lease cost | | | 6,110 | | | 6,659 | | | 11,803 | | | 22,672 |
Total Lease Cost | | | $42,880 | | | $53,788 | | | $85,344 | | | $150,079 |
Year Ending December 31: | | | |
2020 (excluding the six months ended June 30, 2020) | | | $78,437 |
2021 | | | 65,364 |
Minimum lease payments | | | 143,801 |
Less: Imputed interest | | | (7,604) |
Present value of minimum lease payments | | | $136,197 |
8. | Net Loss per Common Share |
9. | Stock-Based Compensation |
| | | For the Three Months Ended June 30, | | | For the Six Months Ended June 30, | |||||||
| | | 2020 | | | 2019 | | | 2020 | | | 2019 | |
Statement of operations line item: | | | | | | | | | ||||
General and administrative | | | $66,351 | | | $119,719 | | | $138,771 | | | $236,398 |
Research and development | | | 2,718 | | | 7,934 | | | 6,600 | | | 52,255 |
Total | | | $69,069 | | | $127,653 | | | $145,371 | | | $288,653 |
| | | Number of Options | | | Weighted Average Exercise Price | | | Weighted Average Remaining Contractual Term | | | Aggregate Intrinsic Value | |
Outstanding, January 1, 2020 | | | 204,574 | | | $35.60 | | | 7.3 years | | | $ — |
Granted | | | — | | | $— | | | | | ||
Exercised | | | — | | | $— | | | | | ||
Expired | | | (2,996) | | | $156.41 | | | | | ||
Cancelled | | | (55,354) | | | $59.54 | | | | | ||
Outstanding, June 30, 2020 | | | 146,224 | | | $24.06 | | | 7.6 years | | | $— |
Exercisable, June 30, 2020 | | | 98,834 | | | $28.65 | | | 7.3 years | | | $— |
| | | 2020 | ||||
| | | Number of Options | | | Weighted Average Fair Value at Grant Date | |
Unvested at January 1, 2020 | | | 81,311 | | | $7.90 |
Granted | | | — | | | $— |
Vested | | | (33,127) | | | $5.53 |
Cancelled | | | (794) | | | $9.12 |
Unvested at June 30, 2020 | | | 47,390 | | | $9.55 |
10. | Warrants |
| | | Number of Warrants: | | | | | ||||||
Warrant Issuance | | | June 30, 2020 | | | December 31, 2019 | | | Exercise Price | | | Expiration Date |
Liability-classified Warrants | | | | | | | | | ||||
November 2015 Investors | | | 104,168 | | | 104,168 | | | $63.60 | | | May 2021 |
November 2015 Placement Agent | | | 279 | | | 279 | | | $63.60 | | | Nov. 2020 |
March 2016 Investors | | | 50,651 | | | 50,651 | | | $50.40 | | | Sept. 2021 |
September 2016 Investors | | | 67,084 | | | 67,084 | | | $36.00 | | | Mar. 2022 |
June 2017 Investors | | | 126,264 | | | 126,264 | | | $48.00 | | | Dec. 2022 |
June 2017 Placement Agent | | | 15,153 | | | 15,153 | | | $49.50 | | | June 2022 |
October 2017 Investors | | | 136,058 | | | 136,058 | | | $34.20 | | | Apr. 2023 |
October 2017 Placement Agent | | | 16,327 | | | 16,327 | | | $36.72 | | | Oct. 2022 |
Total liability classified warrants | | | 515,984 | | | 515,984 | | | | | ||
| | | | | | | | | |||||
Equity-classified Warrants | | | | | | | | | ||||
October 2018 Investors | | | 480,771 | | | 480,771 | | | $20.04 | | | Apr. 2024 |
October 2018 Placement Agent | | | 28,848 | | | 28,848 | | | $19.50 | | | Oct. 2023 |
January 2019 Investors | | | 895,886 | | | 895,886 | | | $9.60 | | | Jan. 2024 |
Total equity-classified warrants | | | 1,405,505 | | | 1,405,505 | | | | | ||
Total outstanding warrants | | | 1,921,489 | | | 1,921,489 | | | | | ||
| | | Number of Warrants | | | | | ||||||
| | | Liability- classified | | | Equity- classified | | | Total | | | Weighted average exercise price | |
Balance, January 1, 2020 | | | 515,984 | | | 1,405,505 | | | 1,921,489 | | | $22.10 |
Issued during the period | | | — | | | — | | | — | | | $— |
Exercised during the period | | | — | | | — | | | — | | | $— |
Expired during the period | | | — | | | — | | | — | | | $— |
Balance, June 30, 2020 | | | 515,984 | | | 1,405,505 | | | 1,921,489 | | | $22.10 |
| | | Fair Value as of: | ||||
| | | June 30, 2020 | | | December 31, 2019 | |
Warrant Issuance: | | | | | ||
November 2015 Investors | | | $3,286 | | | $55 |
November 2015 Placement Agent | | | — | | | — |
March 2016 Investor | | | 6,362 | | | 439 |
September 2016 Investors | | | 28,765 | | | 3,196 |
June 2017 Investors | | | 83,463 | | | 11,736 |
June 2017 Placement Agent | | | 6,348 | | | 845 |
October 2017 Investors | | | 129,299 | | | 23,772 |
October 2017 Placement Agent | | | 11,288 | | | 1,674 |
Total: | | | $268,811 | | | $41,717 |
| | | June 30, 2020 | | | December 31, 2019 | |
Trading market prices | | | $2.84 | | | $1.91 |
Fundamental transaction volatility | | | 134% | | | 102% |
Dividend | | | — | | | — |
Fundamental transaction risk-free rate | | | 0.17-0.21% | | | 1.57-1.72% |
Equivalent volatility | | | 92-103% | | | 85-94% |
Equivalent risk-free rate | | | 0.16% | | | 1.57-1.59% |
Fundamental transaction likelihood | | | 90% | | | 50% |
Fundamental transaction timing | | | September 2020 | | | April 2020 |
| | | For the Three Months Ended June 30, | | | For the Six Months Ended June 30, | |||||||
| | | 2020 | | | 2019 | | | 2020 | | | 2019 | |
November 2015 Investors | | | $(2,315) | | | $27,069 | | | $(3,231) | | | $224,153 |
November 2015 Placement Agent | | | — | | | 55 | | | — | | | 435 |
March 2016 Investors | | | (5,122) | | | 32,874 | | | (5,923) | | | 147,673 |
September 2016 Investors | | | (14,126) | | | 72,213 | | | (25,569) | | | 290,082 |
June 2017 Investors | | | (53,099) | | | 129,559 | | | (71,727) | | | 517,950 |
June 2017 Placement Agent | | | (3,774) | | | 14,553 | | | (5,503) | | | 56,464 |
October 2017 Investors | | | (83,121) | | | 135,125 | | | (105,527) | | | 631,626 |
October 2017 Placement Agent | | | (7,145) | | | 16,035 | | | (9,614) | | | 72,471 |
Total: | | | $(168,702) | | | $427,483 | | | $(227,094) | | | $1,940,854 |
11. | Income Taxes |
| | | June 30, 2020 | | | December 31, 2019 | |
Net Operating Loss Carryforwards | | | $44,843,000 | | | $43,844,000 |
Stock Compensation Expense | | | 540,000 | | | 1,191,000 |
Book Tax Differences on Assets and Liabilities | | | 227,000 | | | 464,000 |
Valuation Allowance | | | (45,610,000) | | | (45,499,000) |
Net Deferred Tax Assets | | | $— | | | $— |
12. | Commitments and Contingencies |
a) | The Company has contracted with various vendors for services, with terms that require payments over the terms of the agreements, usually ranging from two to 36 months. The costs to be incurred are estimated and are subject to revision. As of June 30, 2020, the total estimated cost to complete these agreements was approximately $210,000. All of these agreements may be terminated by either party upon appropriate notice as stipulated in the respective agreements. |
b) | On June 22, 2009, the Company entered into a License Agreement with Korea Research Institute of Chemical Technology (“KRICT”) to acquire the rights to all intellectual property related to quinoxaline-piperazine derivatives that were synthesized under a Joint Research Agreement. The agreement with KRICT calls for a one-time milestone payment of $1,000,000 within 30 days after the first achievement of marketing approval of the first commercial product arising out of or in connection with the use of KRICT’s intellectual property. As of June 30, 2020, the milestone has not occurred. |
c) | The Company has established a 401(k) plan for its employees. The Company has elected to match 100% of the first 3% of an employee’s compensation plus 50% of an additional 2% of the employee’s deferral. Expense related to this matching contribution aggregated to $9,785 and $18,506 for the three months ended June 30, 2020 and 2019, respectively, and $21,014 and $46,385 for the six months ended June 30, 2020 and 2019, respectively. |
d) | On February 5, 2018, the Company and Next BT terminated a research collaboration agreement between the Company and Rexgene Biotech Co., Ltd, a predecessor in interest to Next BT. The Company agreed to pay Next BT a royalty in the low single digits of any net sales of RX-0201 the Company makes in Asia and 50% of the Company’s licensing revenue related to licensing of RX-0201 in Asia, up to an aggregate of $5,000,000. On June 18, 2018, the Company reinstated the exclusive license to RX-0201 in Asia, which had no effect on the potential royalty payments granted to Next BT in February 2018. As of June 30, 2020, the Company has not made any royalty payments to Next BT. |
13. | Fair Value Measurements |
Level 1 Inputs — | Unadjusted quoted prices in active markets for identical assets or liabilities that are accessible by the Company; |
Level 2 Inputs — | Quoted prices in markets that are not active or financial instruments for which all significant inputs are observable, either directly or indirectly; |
Level 3 Inputs — | Unobservable inputs for the asset or liability including significant assumptions of the Company and other market participants. |
Fair Value Measurements at June 30, 2020 | ||||||||||||
| | | Total | | | Level 1 | | | Level 2 | | | Level 3 | |
Liabilities: | | | | | | | | | ||||
Warrant Liabilities | | | $268,811 | | | $— | | | $— | | | $268,811 |
Fair Value Measurements at December 31, 2019 | ||||||||||||
| | | Total | | | Level 1 | | | Level 2 | | | Level 3 | |
Assets: | | | | | | | | | ||||
Commercial Paper | | | $1,997,400 | | | $— | | | $1,997,400 | | | $— |
Corporate Bonds | | | 999,820 | | | — | | | 999,820 | | | — |
Total Assets: | | | $2,997,220 | | | $— | | | $2,997,220 | | | $— |
| | | | | | | | | |||||
Liabilities: | | | | | | | | | ||||
Warrant Liabilities | | | $41,717 | | | $— | | | $— | | | $41,717 |
| | | Warrant Liabilities | |
Balance at January 1, 2020 | | | $41,717 |
Unrealized losses, net | | | 227,094 |
Balance at June 30, 2020 | | | $268,811 |
14. | Subsequent Events |
| | | As of December 31, | ||||
| | | 2019 | | | 2018 | |
Assets | | | | | ||
Current assets: | | | | | ||
Cash | | | $1,536,917 | | | $451,342 |
Prepaids and other assets | | | 149,022 | | | 13,750 |
Deferred costs | | | 76,165 | | | 67,981 |
Total current assets | | | 1,762,104 | | | 533,073 |
Property and equipment, net | | | 22,175 | | | — |
Total assets | | | $1,784,279 | | | $533,073 |
| | | | | |||
Liabilities and stockholders’ deficit | | | | | ||
Current liabilities: | | | | | ||
Accounts payable | | | $341,634 | | | $490,382 |
Accrued expenses | | | 621,671 | | | 98,219 |
Convertible notes | | | 4,977,074 | | | 987,332 |
Convertible notes from related parties | | | 689,756 | | | 351,102 |
Premium conversion derivatives | | | 2,713,668 | | | 304,712 |
Total current liabilities | | | 9,343,803 | | | 2,231,747 |
Total liabilities | | | 9,343,803 | | | 2,231,747 |
| | | | | |||
Commitments and contingencies | | | | | ||
| | | | | |||
Stockholders’ deficit | | | | | ||
Preferred stock, par value $0.0001; 625,000 shares authorized as of December 31, 2019 and 2018; no shares issued and outstanding at December 31, 2019 and 2018. | | | — | | | — |
Common stock, par value $0.0001; 5,000,000 and 3,375,000 shares authorized as of December 31, 2019 and 2018, respectively: 2,700,000 shares issued and outstanding at December 31, 2019 and 2018. | | | 270 | | | 270 |
Additional paid-in-capital | | | 494,909 | | | 186,799 |
Accumulated deficit | | | (8,054,703) | | | (1,885,743) |
Total stockholders’ deficit | | | (7,559,524) | | | (1,698,674) |
Total liabilities and stockholders’ deficit | | | $1,784,279 | | | $533,073 |
| | | For the Year Ended December 31, | ||||
| | | 2019 | | | 2018 | |
Operating expenses: | | | | | ||
General and administrative | | | $1,820,477 | | | $743,279 |
Research and development | | | 2,372,502 | | | 555,951 |
Total operating expenses | | | 4,192,979 | | | 1,299,230 |
Loss from operations | | | (4,192,979) | | | (1,299,230) |
Interest expense | | | (1,409,096) | | | (196,506) |
Fair value change of premium conversion derivative | | | (499,414) | | | (21,238) |
Other expense, net | | | (67,471) | | | (109,897) |
Loss before income taxes | | | (6,168,960) | | | (1,626,871) |
Benefit (provision) for income taxes | | | — | | | — |
Net loss | | | (6,168,960) | | | (1,626,871) |
Other comprehensive loss, net of tax | | | — | | | — |
Comprehensive loss | | | $(6,168,960) | | | $(1,626,871) |
Net loss per share: | | | | | ||
Basic and diluted (Note 9) | | | $(2.29) | | | $(0.68) |
Number of shares used in per share calculations: | | | | | ||
Basic and diluted | | | 2,692,793 | | | 2,388,941 |
| | | Members’ | | | Common Stock | | | Additional Paid–In Capital | | | Accumulated Deficit | | | Total Deficit | ||||
| | | Deficit | | | Shares | | | Amount | | |||||||||
Balance at December 31, 2017 | | | $(254,461) | | | — | | | $— | | | $— | | | $— | | | $(254,461) |
Distribution to related party | | | (4,411) | | | — | | | — | | | — | | | — | | | (4,411) |
Net and comprehensive loss | | | (56,103) | | | — | | | — | | | — | | | — | | | (56,103) |
Effect of merger | | | 314,975 | | | 1,700,003 | | | 170 | | | (170) | | | (314,975) | | | — |
Issuance of restricted stock awards | | | — | | | 999,997 | | | 100 | | | 9,650 | | | — | | | 9,750 |
Share–based compensation | | | — | | | — | | | — | | | 177,319 | | | — | | | 177,319 |
Net and comprehensive loss | | | — | | | — | | | — | | | — | | | (1,570,768) | | | (1,570,768) |
Balance at December 31, 2018 | | | — | | | 2,700,000 | | | 270 | | | 186,799 | | | (1,885,743) | | | (1,698,674) |
Share–based compensation | | | — | | | — | | | — | | | 308,110 | | | — | | | 308,110 |
Net and comprehensive loss | | | — | | | — | | | — | | | — | | | (6,168,960) | | | (6,168,960) |
Balance at December 31, 2019 | | | $— | | | 2,700,000 | | | $270 | | | $494,909 | | | $(8,054,703) | | | $(7,559,524) |
| | | For the Year Ended December 31, | ||||
| | | 2019 | | | 2018 | |
Operating activities | | | | | ||
Net loss | | | $(6,168,960) | | | $(1,626,871) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | ||
Share-based compensation | | | 308,110 | | | 177,319 |
Depreciation | | | 2,762 | | | — |
Non-cash interest on promissory notes – related party | | | — | | | 6,345 |
Non-cash interest on convertible notes | | | 252,225 | | | 39,005 |
Non-cash interest on convertible notes – related party | | | 41,594 | | | 17,126 |
Non-cash discount amortization on convertible notes | | | 1,014,309 | | | 94,526 |
Non-cash discount amortization on convertible notes – related party | | | 100,968 | | | 39,504 |
Fair value change in premium conversion derivatives | | | 499,414 | | | 21,238 |
Change in assets and liabilities: | | | | | ||
Prepaid expenses and other assets | | | 57,710 | | | (10,627) |
Accounts payable | | | (168,098) | | | 380,753 |
Accrued and other liabilities | | | 466,636 | | | 96,315 |
Net cash used in operating activities | | | (3,593,330) | | | (765,367) |
Investing activities | | | | | ||
Purchases of property and equipment | | | (24,937) | | | — |
Net cash used in investing activities | | | (24,937) | | | — |
Financing activities | | | | | ||
Proceeds from issuance of promissory notes – related party | | | — | | | 31,060 |
Proceeds from issuance of convertible notes | | | 4,382,500 | | | 1,050,000 |
Proceeds from issuance of convertible notes – related party | | | 323,040 | | | 150,000 |
Issuance costs attributed to convertible notes | | | (1,698) | | | (12,712) |
Deferred costs | | | — | | | (7,281) |
Distribution to related party | | | — | | | (4,411) |
Issuance of restricted stock awards | | | — | | | 9,750 |
Net cash provided by financing activities | | | 4,703,842 | | | 1,216,406 |
Net increase in cash and cash equivalents | | | 1,085,575 | | | 451,039 |
Cash and cash equivalents at beginning of period | | | 451,342 | | | 303 |
Cash and cash equivalents at end of period | | | $1,536,917 | | | $451,342 |
Supplemental disclosure of cash flow information: | | | | | ||
Cash paid for income taxes | | | $— | | | $— |
Cash paid for interest | | | $— | | | $— |
Supplemental non-cash financing transactions: | | | | | ||
Non-cash conversion of promissory notes to convertible notes | | | $— | | | $244,460 |
Non-cash conversion of advances to promissory notes | | | $— | | | $25,042 |
Bifurcation and modification of premium conversion derivative related to convertible notes | | | $1,909,542 | | | $283,474 |
Unpaid deferred offering costs | | | $76,165 | | | $60,700 |
Proceeds receivable from convertible note issuance | | | $125,000 | | | $— |
• | Level 1 inputs: Unadjusted quoted prices for identical assets or liabilities in active markets; |
• | Level 2 inputs: Quoted prices for similar assets and liabilities in active markets, quoted prices in markets that are not active, or inputs which are observable, whether directly or indirectly, for substantially the full term of the asset or liability; and |
• | Level 3 inputs: Unobservable inputs that reflect the Company’s own assumptions about the assumptions market participants would use in pricing the asset or liability in which there is little, if any, market activity for the asset or liability at the measurement date. |
| | | As of December 31, 2019 | ||||||||||
Description | | | Total | | | Level 1 | | | Level 2 | | | Level 3 |
Liabilities: | | | | | | | | | ||||
Premium conversion derivatives | | | $2,713,668 | | | $ — | | | $ — | | | $2,713,668 |
Total liabilities at fair value | | | $2,713,668 | | | $— | | | $— | | | $2,713,668 |
| | | As of December 31, 2018 | ||||||||||
Description | | | Total | | | Level 1 | | | Level 2 | | | Level 3 |
Liabilities: | | | | | | | | | ||||
Premium conversion derivatives | | | $304,712 | | | $ — | | | $ — | | | $304,712 |
Total liabilities at fair value | | | $304,712 | | | $— | | | $— | | | $304,712 |
| | | 2019 | | | 2018 | |
Premium conversion derivatives | | | | | ||
Balance as of beginning of period | | | $304,712 | | | $— |
Value assigned to the underlying derivatives in connection with convertible notes | | | 1,909,542 | | | 283,474 |
Change in fair value of premium conversion derivatives | | | 499,414 | | | 21,238 |
Balance as of end of period | | | $2,713,668 | | | $304,712 |
• | Removals: the amount of and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy; the policy for timing of transfers between levels; and the valuation processes for Level 3 fair value measurements. |
• | Modifications: for investments in certain entities that calculate net asset value, an entity is required to disclose the timing of liquidation of an investee’s assets and the date when restrictions from redemption might lapse only if the investee has communicated the timing to the entity or announced the timing publicly; and the amendments clarify that the measurement uncertainty disclosure is to communicate information about the uncertainty in measurement as of the reporting date. |
• | Additions: the changes in unrealized gains and losses for the period included in other comprehensive income for recurring Level 3 fair value measurements held at the end of the reporting period; and the range and weighted average of significant unobservable inputs used to develop Level 3 fair value measurements. |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
Prepaids | | | $18,755 | | | $9,950 |
Proceeds receivable from convertible note financing | | | 75,000 | | | — |
Proceeds receivable from convertible note financing – related party | | | 50,000 | | | |
Other | | | 5,267 | | | 3,800 |
Total prepaids and other assets | | | $149,022 | | | $13,750 |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
Equipment | | | $20,175 | | | $ — |
Furniture | | | 4,762 | | | — |
Total property and equipment | | | 24,937 | | | — |
Less accumulated depreciation | | | (2,762) | | | — |
Property and equipment, net | | | $22,175 | | | $— |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
Payroll | | | $350,082 | | | $77,902 |
Professional services | | | 262,397 | | | 18,795 |
Other | | | 9,192 | | | 1,522 |
Total | | | $621,671 | | | $98,219 |
• | IPO: The Convertible Notes will automatically convert into that number of fully paid and non-assessable shares of the Company’s common stock equal to One Hundred and Seventy-Five Percent (175%) times the outstanding principal and accrued but unpaid interest (Note Value) divided by the per share price such shares are issued to purchasers of the Company’s equity securities in the IPO, rounded to the nearest whole share. |
• | CIC: The Convertible Notes will automatically convert immediately prior to the effectiveness of such CIC into that number of fully paid and non-assessable shares of the Company’s common stock equal to Two Hundred Percent (200%) of the Note Value divided by the per share price of the Company’s common stock at which the Company’s common stock is valued in such CIC (after giving effect to such conversion). The Convertible Note holder shall be entitled to the same contractual rights and be bound by the same restrictions and obligations as the other stockholders of the Company in such CIC The terms related to a CIC outside of the conversion formula remain unchanged from those stated in the original Convertible Note agreement as described under – Original Terms, further below. |
• | Qualified Financing: The Convertible Notes will automatically convert into that number of fully paid and non-assessable shares of the Company that are issued by the Company in the Qualified Financing, determined by dividing an amount equal to One Hundred and Seventy-Five Percent (175%) times the Note Value by the per share price such shares of the Company are issued to purchasers of the Company’s equity securities in the Qualified Financing, rounded to the nearest whole share. The Convertible Note holder shall be entitled to the same contractual rights and be bound by the same |
• | Reverse Merger: The Convertible Notes will shall automatically convert into that number of fully paid and non-assessable shares of the combined company whose shares are publicly traded in the United States or other jurisdiction following the completion of the Reverse Merger (the Reverse Merger Parent), determined by dividing an amount equal to One Hundred and Seventy-Five Percent (175%) times the Note Value divided by the per share price at which such shares are issued by the Reverse Merger Parent in such Reverse Merger, rounded to the nearest whole share. The Convertible Note holder shall be entitled to the same contractual rights and be bound by the same restrictions and obligations as the other stockholders of the Company in the Reverse Merger The terms related to a Reverse Merger outside of the conversion formula remain unchanged from those stated in the original Convertible Note agreement as described under – Original Terms, further below. |
| | | December 31, | ||||
| | | 2019 | | | 2018 | |
General and administrative | | | $185,897 | | | $103,157 |
Research and development | | | 122,213 | | | 74,162 |
Total share-based compensation | | | $308,110 | | | $177,319 |
| | | Number of Options | | | Weighted Average Exercise Price | | | Weighted Average Remaining Contractual Term (years) | | | Aggregate Intrinsic Value(1) | |
Outstanding at December 31, 2017 | | | — | | | $— | | | — | | | $— |
Granted | | | 433,719 | | | $0.95 | | | — | | | — |
Exercised | | | — | | | $— | | | — | | | — |
Forfeited/Cancelled | | | — | | | $— | | | — | | | — |
Outstanding at December 31, 2018 | | | 433,719 | | | $0.95 | | | 9.28 | | | $26,023 |
Granted | | | 548,500 | | | $1.26 | | | — | | | — |
Exercised | | | — | | | $— | | | — | | | — |
Forfeited/Cancelled | | | — | | | $— | | | — | | | — |
Outstanding at December 31, 2019 | | | 982,219 | | | $1.12 | | | 9.20 | | | $1,374,324 |
Vested and expected to vest at December 31, 2019 | | | 533,419 | | | $1.00 | | | 8.54 | | | $812,764 |
| | | 2019 | | | 2018 | |
Expected stock price volatility | | | 92.1% | | | 84.7% |
Expected life of options (years) | | | 5.5 | | | 5.0 |
Expected dividend yield | | | 0% | | | 0% |
Risk free interest rate | | | 1.7% | | | 2.9% |
| | | Number of Shares | |
Non-vested at December 31, 2017 | | | — |
Granted | | | 999,997 |
Vested | | | (938,897) |
Non-vested at December 31, 2018 | | | 61,100 |
Granted | | | — |
Vested | | | (61,100) |
Non-vested at December 31, 2019 | | | — |
| | | 2019 | | | 2018 | |
Income tax (benefit) provision at federal statutory rate | | | (21.0)% | | | (21.0)% |
Valuation allowance | | | 24.2 | | | 22.5 |
State income tax, net of federal benefit | | | (4.7) | | | (4.7) |
Stock options | | | 0.2 | | | 1.4 |
Convertible notes | | | 1.2 | | | 0.9 |
Pass through entity and other | | | 0.1 | | | 0.9 |
Effective tax rate | | | —% | | | —% |
| | | 2019 | | | 2018 | |
Deferred tax assets: | | | | | ||
Federal and state operating loss carryforwards | | | $1,228,040 | | | $173,235 |
Research and development costs deferral election | | | — | | | 114,475 |
Accruals | | | 87,310 | | | 16,359 |
Convertible notes | | | 453,939 | | | 38,318 |
Organizational costs | | | 8,425 | | | 9,055 |
Stock-based compensation | | | 81,397 | | | 16,000 |
Subtotal | | | 1,859,111 | | | 367,442 |
Valuation allowance | | | (1,859,111) | | | (367,442) |
Total deferred tax assets, net of valuation allowance | | | — | | | — |
Deferred tax liabilities: | | | | | ||
Total deferred tax liabilities | | | — | | | — |
Net deferred tax assets | | | $— | | | $— |
| | | 2019 | | | 2018 | |
Stock options | | | 982,219 | | | 433,719 |
Restricted stock awards | | | — | | | 61,100 |
| | | June 30, 2020 (Unaudited) | | | December 31, 2019 | |
Assets | | | | | ||
Current assets: | | | | | ||
Cash and cash equivalents | | | $854,331 | | | $1,536,917 |
Proceeds receivable from convertible notes | | | 1,425,000 | | | 75,000 |
Proceeds receivable from convertible notes - related parties | | | — | | | 50,000 |
Prepaids and other assets | | | 23,439 | | | 24,022 |
Deferred costs | | | 1,181,334 | | | 76,165 |
Total current assets | | | 3,484,104 | | | 1,762,104 |
Property and equipment, net | | | 15,804 | | | 22,175 |
Total assets | | | $3,499,908 | | | $1,784,279 |
| | | | | |||
Liabilities and stockholders’ deficit | | | | | ||
Current liabilities: | | | | | ||
Accounts payable | | | $671,278 | | | $341,634 |
Accrued liabilities | | | 1,305,390 | | | 621,671 |
Convertible notes | | | 8,282,551 | | | 4,977,074 |
Convertible notes from related parties | | | 838,277 | | | 689,756 |
Premium conversion derivatives | | | 1,179,765 | | | 2,713,668 |
Total current liabilities | | | 12,277,261 | | | 9,343,803 |
Total liabilities | | | 12,277,261 | | | 9,343,803 |
| | | | | |||
Commitments and contingencies (Note 2) | | | | | ||
| | | | | |||
Stockholders’ deficit | | | | | ||
Preferred stock, par value $0.0001; 625,000 shares authorized as of June 30, 2020 and December 31, 2019; no shares issued and outstanding at June 30, 2020 and December 31, 2019. | | | — | | | — |
Common stock, par value $0.0001; 5,000,000 shares authorized as of June 30, 2020 and December 31, 2019; 3,543,751 and 2,700,000 shares issued and outstanding at June 30, 2020 and December 31, 2019, respectively. | | | 354 | | | 270 |
Additional paid-in-capital | | | 3,969,494 | | | 494,909 |
Accumulated deficit | | | (12,747,201) | | | (8,054,703) |
Total stockholders’ deficit | | | (8,777,353) | | | (7,559,524) |
Total liabilities and stockholders’ deficit | | | $3,499,908 | | | $1,784,279 |
| | | For the Three Months Ended June 30, | | | For the Six Months Ended June 30, | |||||||
| | | 2020 | | | 2019 | | | 2020 | | | 2019 | |
Operating expenses: | | | | | | | | | ||||
General and administrative | | | $551,391 | | | $414,061 | | | $942,471 | | | $777,189 |
Research and development | | | 710,752 | | | 450,885 | | | 928,561 | | | 828,450 |
Acquired in-process research and development | | | — | | | — | | | 2,126,253 | | | — |
Total operating expenses | | | 1,262,143 | | | 864,946 | | | 3,997,285 | | | 1,605,639 |
Loss from operations | | | (1,262,143) | | | (864,946) | | | (3,997,285) | | | (1,605,639) |
Interest expense | | | (688,865) | | | (213,928) | | | (1,242,624) | | | (319,869) |
Fair value change of premium conversion derivatives | | | (919,409) | | | (34,653) | | | (721,444) | | | (132,083) |
Gain on note extinguishment (Note 4) | | | 1,260,350 | | | — | | | 1,260,350 | | | — |
Other income, net | | | 5,885 | | | — | | | 8,505 | | | — |
Loss before income taxes | | | (1,604,182) | | | (1,113,527) | | | (4,692,498) | | | (2,057,591) |
Benefit (provision) for income taxes | | | — | | | — | | | — | | | — |
Net loss | | | (1,604,182) | | | (1,113,527) | | | (4,692,498) | | | (2,057,591) |
Other comprehensive loss, net of tax | | | — | | | — | | | — | | | — |
Comprehensive loss | | | $(1,604,182) | | | $(1,113,527) | | | $(4,692,498) | | | $(2,057,591) |
Net loss per share: Basic and diluted (Note 9) | | | $($0.45) | | | $($0.41) | | | $(1.36) | | | $(0.77) |
Number of shares used in per share calculations: Basic and diluted | | | 3,543,751 | | | 2,699,330 | | | 3,451,031 | | | 2,685,467 |
| | | Common Stock | | | Additional Paid–In Capital | | | Accumulated Deficit | | | Total Deficit | ||||
| | | Shares | | | Amount | | |||||||||
Balance at December 31, 2018 | | | 2,700,000 | | | $270 | | | $186,799 | | | $(1,885,743) | | | $(1,698,674) |
Share–based compensation – employee | | | — | | | — | | | 65,343 | | | — | | | 65,343 |
Share–based compensation – non-employee | | | — | | | — | | | 20,519 | | | — | | | 20,519 |
Net and comprehensive loss | | | — | | | — | | | — | | | (944,064) | | | (944,064) |
Balance at March 31, 2019 | | | 2,700,000 | | | 270 | | | 272,661 | | | (2,829,807) | | | (2,556,876) |
Share–based compensation – employee | | | — | | | — | | | 52,594 | | | — | | | 52,594 |
Share–based compensation – non-employee | | | — | | | — | | | 18,030 | | | — | | | 18,030 |
Net and comprehensive loss | | | — | | | — | | | — | | | (1,113,527) | | | (1,113,527) |
Balance at June 30, 2019 | | | 2,700,000 | | | $270 | | | $343,285 | | | $(3,943,334) | | | $(3,599,779) |
Balance at December 31, 2019 | | | 2,700,000 | | | $270 | | | $494,909 | | | $(8,054,703) | | | $(7,559,524) |
Issuance of common stock in exchange for in-process research and development | | | 843,751 | | | 84 | | | 2,126,169 | | | — | | | 2,126,253 |
Share–based compensation | | | — | | | — | | | 61,324 | | | — | | | 61,324 |
Net and comprehensive loss | | | — | | | — | | | — | | | (3,088,316) | | | (3,088,316) |
Balance at March 31, 2020 | | | 3,543,751 | | | 354 | | | $2,682,402 | | | (11,143,019) | | | (8,460,263) |
Gain on note extinguishment (Note 4) | | | — | | | — | | | 970,628 | | | — | | | 970,628 |
Share–based compensation | | | — | | | — | | | 316,464 | | | — | | | 316,464 |
Net and comprehensive loss | | | — | | | — | | | — | | | (1,604,182) | | | (1,604,182) |
Balance at June 30, 2020 | | | 3,543,751 | | | $354 | | | $3,969,494 | | | $(12,747,201) | | | $(8,777,353) |
| | | Six Months Ended June 30, | ||||
| | | 2020 | | | 2019 | |
Operating activities | | | | | ||
Net loss | | | $(4,692,498) | | | $(2,057,591) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | ||
Share-based compensation | | | 377,788 | | | 156,486 |
Depreciation | | | 6,371 | | | 359 |
Non-cash acquired in-process research and development | | | 2,126,253 | | | — |
Non-cash interest on convertible notes | | | 276,782 | | | 68,129 |
Non-cash interest on convertible notes – related party | | | 29,802 | | | 17,661 |
Non-cash discount amortization on convertible notes | | | 865,313 | | | 204,381 |
Non-cash discount amortization on convertible notes – related party | | | 70,727 | | | 29,698 |
Fair value change in premium conversion derivatives | | | 721,444 | | | 132,083 |
Gain on note extinguishment | | | (1,260,350) | | | — |
Change in assets and liabilities: | | | | | ||
Prepaid expenses and other assets | | | 125,582 | | | (21,472) |
Accounts payable | | | 177,946 | | | (83,957) |
Accrued and other liabilities | | | (208,461) | | | 240,513 |
Net cash used in operating activities | | | (1,383,301) | | | (1,313,710) |
Investing activities | | | | | ||
Purchases of property and equipment | | | — | | | (20,175) |
Net cash used in investing activities | | | — | | | (20,175) |
Financing activities | | | | | ||
Proceeds from issuance of convertible notes | | | 772,500 | | | 2,000,000 |
Proceeds from issuance of convertible notes – related party | | | — | | | 75,000 |
Deferred offering costs | | | (71,785) | | | — |
Issuance costs attributed to convertible notes | | | — | | | (663) |
Net cash provided by financing activities | | | 700,715 | | | 2,074,337 |
Net (decrease) increase in cash and cash equivalents | | | (682,586) | | | 740,452 |
Cash and cash equivalents at beginning of period | | | 1,536,917 | | | 451,342 |
Cash and cash equivalents at end of period | | | $854,331 | | | $1,191,794 |
Supplemental disclosure of cash flow information: | | | | | ||
Cash paid for income taxes | | | $— | | | $— |
Cash paid for interest | | | $— | | | $— |
Supplemental non-cash financing transactions: | | | | | ||
Bifurcation and modification of premium conversion derivatives related to convertible notes | | | $831,172 | | | $1,099,609 |
Purchases of property and equipment in accrued liabilities | | | $— | | | $1,378 |
Unpaid deferred offering and issuance costs | | | $1,043,879 | | | $1,035 |
Proceeds receivable from convertible note issuance | | | $1,425,000 | | | $1,680,540 |
• | Level 1 inputs: Unadjusted quoted prices for identical assets or liabilities in active markets; |
• | Level 2 inputs: Quoted prices for similar assets and liabilities in active markets, quoted prices in markets that are not active, or inputs which are observable, whether directly or indirectly, for substantially the full term of the asset or liability; and |
• | Level 3 inputs: Unobservable inputs that reflect the Company’s own assumptions about the assumptions market participants would use in pricing the asset or liability in which there is little, if any, market activity for the asset or liability at the measurement date. |
| | | As of June 30, 2020 | ||||||||||
Description | | | Total | | | Level 1 | | | Level 2 | | | Level 3 |
Liabilities: | | | | | | | | | ||||
Premium conversion derivatives | | | $1,179,765 | | | $— | | | $— | | | $1,179,765 |
Total liabilities at fair value | | | $1,179,765 | | | $— | | | $— | | | $1,179,765 |
| | | As of December 31, 2019 | ||||||||||
Description | | | Total | | | Level 1 | | | Level 2 | | | Level 3 |
Liabilities: | | | | | | | | | ||||
Premium conversion derivatives | | | $2,713,668 | | | $— | | | $— | | | $2,713,668 |
Total liabilities at fair value | | | $2,713,668 | | | $— | | | $— | | | $2,713,668 |
| | | 2020 | | | 2019 | |
Premium conversion derivatives | | | | | ||
Balance as of beginning of period | | | $2,713,668 | | | $304,712 |
Value assigned to the underlying derivatives in connection with convertible notes | | | 831,172 | | | 1,099,609 |
Revaluation due to convertible note extinguishment | | | (3,086,519) | | | — |
Change in fair value of premium conversion derivatives | | | 721,444 | | | 132,083 |
Balance as of end of period | | | $1,179,765 | | | $1,536,404 |
• | Removals: the amount of and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy; the policy for timing of transfers between levels; and the valuation processes for Level 3 fair value measurements. |
• | Modifications: for investments in certain entities that calculate net asset value, an entity is required to disclose the timing of liquidation of an investee’s assets and the date when restrictions from redemption might lapse only if the investee has communicated the timing to the entity or announced the timing publicly; and the amendments clarify that the measurement uncertainty disclosure is to communicate information about the uncertainty in measurement as of the reporting date. |
• | Additions: the changes in unrealized gains and losses for the period included in other comprehensive income for recurring Level 3 fair value measurements held at the end of the reporting period; and the range and weighted average of significant unobservable inputs used to develop Level 3 fair value measurements. |
| | | June 30, 2020 | | | December31, 2019 | |
Equipment | | | $20,175 | | | $20,175 |
Furniture | | | 4,762 | | | 4,762 |
Total property and equipment | | | 24,937 | | | 24,937 |
Less accumulated depreciation | | | (9,133) | | | (2,762) |
Property and equipment, net | | | $15,804 | | | $22,175 |
| | | June 30, 2020 | | | December 31, 2019 | |
Deferred offering and issuance costs | | | $897,205 | | | $— |
Payroll | | | 356,217 | | | 350,082 |
Professional services | | | 50,000 | | | 262,397 |
Other | | | 1,968 | | | 9,192 |
Total | | | $1,305,390 | | | $621,671 |
• | IPO: The Convertible Notes will automatically convert into the number of fully paid and non-assessable shares of the Company’s common stock equal to One Hundred and Seventy-Five Percent (175%) times the Note Value divided by the per share price such shares are issued to purchasers of the Company’s equity securities in the IPO rounded to the nearest whole share. |
• | CIC: The Convertible Notes will automatically convert immediately prior to the effectiveness of such CIC into that number of fully paid and non-assessable shares of the Company’s common stock equal to Two Hundred Percent (200%) of the Note Value divided by the per share price of the Company’s common stock at which the Company’s common stock is valued in such CIC (after giving effect to such conversion). The Convertible Note holder shall be entitled to the same contractual rights and be bound by the same restrictions and obligations as the other stockholders of the Company in such CIC. |
• | Qualified Financing: The Convertible Notes will automatically convert into that number of fully paid and non-assessable shares of the Company that are issued by the Company in the Qualified Financing, determined by dividing an amount equal to One Hundred and Seventy-Five Percent (175%) times the Note Value by the per share price such shares of the Company are issued to purchasers of the Company’s equity securities in the Qualified Financing, rounded to the nearest whole share. The Convertible Note holder shall be entitled to the same contractual rights and be bound by the same restrictions and obligations as the other purchasers of shares in the Qualified Financing. A Qualified Financing is defined as a sale and issuance of capital stock of the Company (or its successor) in a single transaction or series of related transactions resulting in gross proceeds to the Company of not less than $5,000,000 (including new equity investment of at least $1,000,000 plus the sum of the outstanding principal amount of the Convertible Notes being so converted under this provision). |
• | Reverse Merger (excluding close of Merger with Rexahn): The Convertible Notes will shall automatically convert into that number of fully paid and non-assessable shares of the Combined Company whose shares are publicly traded in the United States or other jurisdiction following the completion of the Reverse Merger (the Reverse Merger Parent), determined by dividing an amount equal to One Hundred and Seventy-Five Percent (175%) times the Note Value divided by the per share price at which such shares are issued by the Reverse Merger Parent in such Reverse Merger, rounded to the nearest whole share. The Convertible Note holder shall be entitled to the same contractual rights and be bound by the same restrictions and obligations as the other stockholders of the Company in the Reverse Merger. |
• | Close of Merger with Rexahn – as defined above under the Conversion Agreement. |
| | | Three Months Ended June 30, | | | Six Months Ended June 30, | |||||||
| | | 2020 | | | 2019 | | | 2020 | | | 2019 | |
General and administrative | | | $159,061 | | | $42,397 | | | $201,461 | | | $90,225 |
Research and development | | | 157,403 | | | 28,227 | | | 176,327 | | | 66,261 |
Total share-based compensation | | | $316,464 | | | $70,624 | | | $377,788 | | | $156,486 |
| | | Three Months Ended June 30, | | | Six Months Ended June 30, | |||||||
| | | 2020 | | | 2019 | | | 2020 | | | 2019 | |
Expected stock price volatility | | | 95.5% | | | 85.8% | | | 95.5% | | | 85.3% |
Expected life of options (years) | | | 10.0 | | | 5.3 | | | 10.0 | | | 5.2 |
Expected dividend yield | | | 0% | | | 0% | | | 0% | | | 0% |
Risk free interest rate | | | 0.7% | | | 1.7% | | | 0.7% | | | 2.1% |
| | | 2020 | | | 2019 | |
Stock options | | | 1,175,000 | | | 481,219 |
| | | | | | | Page | |||
| | | | | ||||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | ||||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | ||||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | Page | |||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | ||||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | ||||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | Page | |||
| | | | | ||||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | ||||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | ||||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | ||||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | ||||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
| | | | | | | ||||
Exhibit A | | | Definitions |
Exhibit B | | | Form of Company Stockholder Support Agreement |
Exhibit C-1 | | | Form of Company Lock-Up Agreement |
Exhibit C-2 | | | Form of Parent Lock-Up Agreement |
Exhibit D | | | Form of Contingent Value Right Agreement |
Exhibit E | | | Post-Closing Directors and Officers |
Exhibit F | | | Form of 2020 Plan |
DESCRIPTION OF TRANSACTION |
REPRESENTATIONS AND WARRANTIES OF THE COMPANY |
REPRESENTATIONS AND WARRANTIES OF PARENT AND MERGER SUB |
CERTAIN COVENANTS OF THE PARTIES |
ADDITIONAL AGREEMENTS OF THE PARTIES |
CONDITIONS PRECEDENT TO OBLIGATIONS OF EACH PARTY |
ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATIONS OF PARENT AND MERGER SUB |
ADDITIONAL CONDITIONS PRECEDENT TO OBLIGATION OF THE COMPANY |
TERMINATION |
MISCELLANEOUS PROVISIONS |
| | | REXAHN PHARMACEUTICALS, INC. | ||||
| | | | | |||
| | | By: | | | /s/ Douglas J. Swirsky | |
| | | Name: | | | Douglas J. Swirsky | |
| | | Title: | | | President and Chief Executive Officer | |
| | | RAZOR MERGER SUB, INC. | ||||
| | | | | |||
| | | By: | | | /s/ Douglas J. Swirsky | |
| | | Name: | | | Douglas J. Swirsky | |
| | | Title: | | | President | |
| | | OCUPHIRE PHARMA, INC. | ||||
| | | | | |||
| | | By: | | | /s/ Mina Sooch | |
| | | Name: | | | Mina Sooch | |
| | | Title: | | | Chief Executive Officer | |
• | “Aggregate Valuation” means the sum of (i) the Company Valuation, plus (ii) the Parent Valuation. |
• | “Company Allocation Percentage” means the quotient (expressed as a percentage with the percentage rounded to two decimal places) determined by dividing (i) the Company Valuation by (ii) the Aggregate Valuation. |
• | “Company Merger Shares” means the product determined by multiplying (i) the Post-Closing Parent Shares by (ii) the Company Allocation Percentage. |
• | “Company Outstanding Shares” means the total number of shares of Company Capital Stock outstanding immediately prior to the Effective Time expressed on a fully-diluted and as-converted to Company Common Stock basis and assuming, without limitation or duplication, (i) the exercise of all Company Options outstanding as of immediately prior to the Effective Time, (ii) the conversion of all Company Convertible Notes and other outstanding indebtedness, (iii) the closing of the Pre-Closing Financing (excluding any shares of Company Common Stock issued into escrow pursuant to the terms of the Pre-Closing Financing), and (iv) the issuance of shares of Company Common Stock in respect of all other outstanding options, restricted stock awards, warrants or rights to receive such shares, whether conditional or unconditional, and including any outstanding options, restricted stock awards, warrants or rights triggered by or associated with the consummation of the Merger (but excluding any other shares of Company Common Stock reserved for issuance under the Company Plan). |
• | “Company Valuation” means $120,000,000. |
• | “Parent Allocation Percentage” means the quotient (expressed as a percentage, with the percentage rounded to two decimal places) determined by dividing (i) the Parent Valuation by (ii) the Aggregate Valuation. |
• | “Parent Outstanding Shares” means the total number of shares of Parent Common Stock outstanding immediately prior to the Effective Time expressed on a fully-diluted and as converted to Parent Common Stock basis, with any in-the-money Replacement Warrants calculated based on the treasury stock method using the Market Price, and (i) assuming, without limitation or duplication, the exercise of all Replacement Warrants (other than Cash Liability Replacement Warrants) (subject to sub-clause |
• | “Parent Valuation” means $20,000,000 (the “Parent Base Valuation”); provided, however, to the extent that (i) the Parent Cash Amount determined pursuant to Section 1.12 is less than $3,200,000, then the Parent Base Valuation shall be reduced by $150,000 for each $100,000 that the Parent Cash Amount as so determined in less than $3,200,000, subject to a minimum Parent Valuation of $12,000,000 (for example, the Parent Valuation would be $19,700,000 if the Parent Cash Amount determined pursuant to Section 1.12 is $3,000,000); and (ii) the Parent Cash Amount determined pursuant to Section 1.12 is greater than $6,000,000, then the Parent Base Valuation shall be increased by $150,000 for each $100,000 that the Parent Cash Amount as so determined is greater than $6,000,000 (for example, the Parent Valuation would be $20,300,000 if the Parent Cash Amount determined pursuant to Section 1.12 is $6,200,000). |
• | “Post-Closing Parent Shares” means the quotient determined by dividing (i) the Parent Outstanding Shares by (ii) the Parent Allocation Percentage. |
Term | | | Section |
2020 Plan | | | 5.3(a)(iv) |
Accounting Firm | | | 1.12(e) |
Agreement | | | Preamble |
Allocation Certificate | | | 5.15(a) |
Anti-Bribery Laws | | | 2.23 |
Anticipated Closing Date | | | 1.12(a) |
Book-Entry Shares | | | 1.6 |
Business Associate Agreements | | | 2.14(i) |
Certificate of Merger | | | 1.3 |
Certifications | | | 3.7(a) |
Closing | | | 1.3 |
Closing Date | | | 1.3 |
Company | | | Preamble |
Company Benefit Plan | | | 2.17(a) |
Company Board Adverse Recommendation Change | | | 5.2(d) |
Company Board Recommendation | | | 5.2(d) |
Company Budget | | | 4.2(b)(v) |
Company Counsel | | | 5.1(c) |
Company Determination Notice | | | 5.2(e)(i) |
Company Disclosure Schedule | | | Section 2 |
Company Financial Statements | | | 2.7(a) |
Company In-bound License | | | 2.12(d) |
Company Interim Financial Statements | | | 5.16 |
Company Lock-Up Agreement | | | Recitals |
Company Material Contract | | | 2.13(a) |
Company Material Contracts | | | 2.13(a) |
Company Out-bound License | | | 2.12(d) |
Company Permits | | | 2.14(c) |
Company Real Estate Leases | | | 2.11 |
Company Registered IP | | | 2.12(a) |
Term | | | Section |
Company Regulatory Permits | | | 2.14(e) |
Company Signatories | | | Recitals |
Company Stock Certificate | | | 1.6 |
Company Stockholder Matters | | | 5.2(a) |
Company Stockholder Support Agreement | | | Recitals |
Company Stockholder Written Consent | | | 2.4 |
Company Termination Fee | | | 9.3(b) |
Convertible Note Conversion | | | 5.21 |
CVR | | | 1.7(a) |
CVR Agreement | | | 1.7(a) |
D&O Indemnified Parties | | | 5.5(a) |
D&O Tail Policy | | | 5.5(d) |
Determination Date | | | 1.12(a) |
Dispute Notice | | | 1.12(b) |
Dissenting Shares | | | 1.9(a) |
Drug Regulatory Agency | | | 2.14(a) |
Effective Time | | | 1.3 |
End Date | | | 9.1(b) |
Exchange Agent | | | 1.8(a) |
Exchange Fund | | | 1.8(a) |
FDA | | | 2.14(a) |
FDCA | | | 2.14(a) |
FLSA | | | 2.17(o) |
GCP | | | 2.14(f) |
GLP | | | 2.14(f) |
HIPAA | | | 2.14(i) |
Information Statement | | | 5.2(a) |
Intended Tax Treatment | | | 5.9(a) |
Investor Agreements | | | 2.22(b) |
Liability | | | 2.9 |
Merger | | | Recitals |
Merger Consideration | | | 1.5(a)(ii) |
Merger Sub | | | Preamble |
Nasdaq Fees | | | 5.8 |
Nasdaq Listing Application | | | 5.8 |
Parent | | | Preamble |
Parent Benefit Plan | | | 3.17(a) |
Parent Board Adverse Recommendation Change | | | 5.3(c) |
Parent Board Recommendation | | | 5.3(c) |
Parent Budget | | | 4.1(b)(v) |
Parent Cash Calculation | | | 1.12(a) |
Parent Cash Schedule | | | 1.12(a) |
Parent Counsel | | | 5.1(c) |
Parent Designee | | | 5.11(a) |
Parent Determination Notice | | | 5.3(d)(i) |
Parent Disclosure Schedule | | | Section 3 |
Parent In-bound License | | | 3.12(d) |
Parent Lock-Up Agreement | | | Recitals |
Parent Material Contract | | | 3.13(a) |
Parent Material Contracts | | | 3.13(a) |
Term | | | Section |
Parent Out-bound License | | | 3.12(d) |
Parent Outstanding Shares Certificate | | | 5.15(b) |
Parent Permits | | | 3.14(c) |
Parent Real Estate Leases | | | 3.11 |
Parent Registered IP | | | 3.12(a) |
Parent Regulatory Permits | | | 3.14(e) |
Parent SEC Documents | | | 3.7(a) |
Parent Signatories | | | Recitals |
Parent Stockholder Matters | | | 5.3(a)(vi) |
Parent Stockholders’ Meeting | | | 5.3(a)(vi) |
Parent Termination Fee | | | 9.3(c) |
Parent Voting Debt | | | 3.6(d) |
PHSA | | | 2.14(a) |
Pre-Closing Period | | | 4.1(a) |
Required Company Stockholder Vote | | | 2.4 |
Required Parent Stockholder Vote | | | 3.4 |
Response Date | | | 1.12(b) |
Sensitive Data | | | 2.12(g) |
Stockholder Notice | | | 5.2(c) |
Surviving Corporation | | | 1.1 |
Terminated Parent Associate | | | 5.20(a) |
Third-Party Expenses | | | 9.3(d) |
| | | OCUPHIRE PHARMA, INC. | |||||||
| | | | | | | ||||
| | | By: | | | |||||
| | | | | Name: | | | |||
| | | | | Title: | | | |||
| | | REXAHN PHARMACEUTICALS, INC. | |||||||
| | | | | | |||||
| | | By: | | | |||||
| | | | | Name: | | | Douglas J. Swirsky | ||
| | | | | Title: | | | President and Chief Executive Officer | ||
| | | STOCKHOLDER | |||||||
| | | | | | | ||||
| | | By: | | | |||||
| | | | | Name: | | | |||
| | | | | Title: | | | |||
| | | | | | |||||
| | | | | Address: | | | |||
| | | | | | |||||
| | | | | Electronic Mail Address: | |||||
Name and Address of Stockholder | | | Number of Shares of Common Stock |
[•] | | | [•] |
| | | Very truly yours, | ||||
| | | | | |||
| | | |||||
| | | Printed Name of Holder | ||||
| | | | | |||
| | | By: | | | ||
| | | | | Signature | ||
| | | | | |||
| | | |||||
| | | Printed Name of Person Signing (and indicate capacity of person signing if signing as custodian, trustee, or on behalf of an entity) | ||||
| | | Very truly yours, | ||||
| | | | | |||
| | | |||||
| | | Printed Name of Holder | ||||
| | | | | |||
| | | By: | | | ||
| | | | | Signature | ||
| | | | | |||
| | | |||||
| | | Printed Name of Person Signing (and indicate capacity of person signing if signing as custodian, trustee, or on behalf of an entity) | ||||
• | Mina Sooch, Chief Executive Officer, President & Treasurer |
• | Bernhard Hoffman, VP of Corporate Development & Finance, Secretary |
• | Mina Sooch |
• | Sean Ainsworth |
• | Alan Meyer |
• | James Manuso |
• | Cam Gallagher |
• | Richard Rodgers |
• | One additional director to be appointed by Ocuphire |
Section A | | | Company Signatories |
Section 2.1(c) | | | Subsidiaries |
Section 2.5 | | | Consents |
Section 2.6(a)(i) | | | Holders of Record of Company Common Stock |
Section 2.6(a)(ii) | | | Company Convertible Note Holders |
Section 2.6(b) | | | Repurchase Rights |
Section 2.6(c) | | | Company Options |
Section 2.7(c) | | | Off-Balance Sheet Arrangements |
Section 2.9 | | | Absence of Undisclosed Liabilities |
Section 2.12(a) | | | Intellectual Property |
Section 2.12(b) | | | Encumbrances on Intellectual Property |
Section 2.12(d) | | | License Agreements |
Section 2.13(a) | | | Contracts |
Section 2.14(c) | | | Company Permits |
Section 2.17(a) | | | Employee Benefit Plans |
Section 2.17(i) | | | Effect of Transaction |
Section 2.20 | | | No Financial Advisors |
Section 2.22(a) | | | Transactions with Affiliates |
Section 2.22(b) | | | Investor Agreements |
Section 4.2 | | | Operation of the Company’s Business |
Section A | | | Parent Signatories |
Section B | | | Parent Warrants |
Section C | | | Estimated Warrant Schedule |
Section 1.12(a) | | | Parent Cash Amount |
Section 3.5 | | | Non-Contravention; Consents |
Section 3.6(b) | | | Capitalization; Repurchase Rights |
Section 3.6(c) | | | Capitalization |
Section 3.6(d) | | | Capitalization |
Section 3.7(d) | | | SEC Filings; Financial Statements |
Section 3.8 | | | Absence of Changes |
Section 3.9 | | | Absence of Undisclosed Liabilities |
Section 3.12(a) | | | Intellectual Property |
Section 3.12(c) | | | Intellectual Property |
Section 3.12(d) | | | Intellectual Property |
Section 3.12(f) | | | Intellectual Property |
Section 3.13(a) | | | Agreements, Contracts and Commitments |
Section 3.13(b) | | | Agreements, Contracts and Commitments |
Section 3.14(c) | | | Compliance; Permits |
Section 3.14(f) | | | Compliance; Permits |
Section 3.17(a) | | | Employee and Labor Matters; Benefit Plans |
Section 3.17(i) | | | Employee and Labor Matters; Benefit Plans |
Section 3.21 | | | Transactions with Affiliates |
Section 4.1(a) | | | Operation of Parent’s Business |
Section 4.1(b) | | | Operation of Parent’s Business |
Section 5.20(a) | | | Employee Benefits |
| | |
| | | REXAHN PHARMACEUTICALS, INC. | ||||
| | | | | |||
| | | By: | | | /s/ Douglas J. Swirsky | |
| | | Name: | | | Douglas J. Swirsky | |
| | | Title: | | | President and Chief Executive Officer | |
| | | RAZOR MERGER SUB, INC. | ||||
| | | | | |||
| | | By: | | | /s/ Douglas J. Swirsky | |
| | | Name: | | | Douglas J. Swirsky | |
| | | Title: | | | President | |
| | | OCUPHIRE PHARMA, INC. | ||||
| | | | | |||
| | | By: | | | /s/ Mina Sooch | |
| | | Name: | | | Mina Sooch | |
| | | Title: | | | Chief Executive Officer | |
| | | REXAHN PHARMACEUTICALS, INC. | ||||
| | | | | |||
| | | By: | | | ||
| | | Name: | | | Douglas J. Swirsky | |
| | | Title: | | | President and Chief Executive Officer | |
| | | REXAHN PHARMACEUTICALS, INC. | ||||
| | | | | |||
| | | By: | | | ||
| | | Name: | | | Douglas J. Swirsky | |
| | | Title: | | | President and Chief Executive Officer | |
 | | | Oppenheimer & Co. Inc. 85 Broad Street 25th Floor New York, NY 10004 Phone 212-668-8000 Transacts Business on All Principal Exchanges |
(i) | reviewed the financial terms of the Merger described in the Draft Merger Agreement and a draft of the CVR Agreement, dated June 17, 2020, that would be executed in connection with the consummation of the Merger. Both the Draft Merger Agreement and the draft of the CVR Agreement were the most recent drafts made available to us prior to delivery of our opinion; |
(ii) | reviewed certain information, including certain projected financial information, relating to the business, earnings, and prospects of Rexahn and the Company that was furnished to us by Rexahn and the Company; |
(iii) | conducted discussions with members of senior management and representatives of Rexahn and the Company concerning the matters described in clause (ii) above; |
(iv) | reviewed the pro forma ownership structure of the combined entity resulting from the Merger; |
(v) | reviewed publicly available information relating to the businesses of Rexahn and the Company; |
(vi) | reviewed and analyzed certain publicly available information concerning the terms (including financial terms) of selected merger and acquisition transactions and other business combinations that we considered relevant to our analysis; |
(vii) | reviewed and analyzed certain publicly available information relating to selected companies that we deemed relevant to our analysis; |
(viii) | performed a discounted cash flow analysis of the future cash flows of the Company based upon financial projections for the Company prepared by management of the Company and approved for our use for such purpose by management of Rexahn (the “Company Projections”); |
(ix) | reviewed the latest Pre-Closing Financing term sheets resulting from a marketed process of the contemplated Pre-Closing Financing conducted by Canaccord Genuity Group Inc. and Cantor Fitzgerald, L.P.; |
(x) | reviewed such other information, performed such other analyses, financial studies and investigations, and considered such other factors as we deemed appropriate for the purpose of rendering our opinion; and |
(xi) | taken into account our assessment of general economic, market and financial conditions and our experience in other transactions, as well as our experience in securities valuations and our knowledge of Rexahn’s and the Company’s industries. |
(a) | Any stockholder of a corporation of this State who holds shares of stock on the date of the making of a demand pursuant to subsection (d) of this section with respect to such shares, who continuously holds such shares through the effective date of the merger or consolidation, who has otherwise complied with subsection (d) of this section and who has neither voted in favor of the merger or consolidation nor consented thereto in writing pursuant to § 228 of this title shall be entitled to an appraisal by the Court of Chancery of the fair value of the stockholder’s shares of stock under the circumstances described in subsections (b) and (c) of this section. As used in this section, the word “stockholder” means a holder of record of stock in a corporation; the words “stock” and “share” mean and include what is ordinarily meant by those words; and the words “depository receipt” mean a receipt or other instrument issued by a depository representing an interest in 1 or more shares, or fractions thereof, solely of stock of a corporation, which stock is deposited with the depository. |
(b) | Appraisal rights shall be available for the shares of any class or series of stock of a constituent corporation in a merger or consolidation to be effected pursuant to § 251 (other than a merger effected pursuant to § 251(g) of this title), § 252, § 254, § 255, § 256, § 257, § 258, § 263 or § 264 of this title: |
1. | Provided, however, that, except as expressly provided in § 363(b) of this title, no appraisal rights under this section shall be available for the shares of any class or series of stock, which stock, or depository receipts in respect thereof, at the record date fixed to determine the stockholders entitled to receive notice of the meeting of stockholders to act upon the agreement of merger or consolidation (or, in the case of a merger pursuant to § 251(h), as of immediately prior to the execution of the agreement of merger), were either: (i) listed on a national securities exchange or (ii) held of record by more than 2,000 holders; and further provided that no appraisal rights shall be available for any shares of stock of the constituent corporation surviving a merger if the merger did not require for its approval the vote of the stockholders of the surviving corporation as provided in § 251(f) of this title. |
2. | Notwithstanding paragraph (b)(1) of this section, appraisal rights under this section shall be available for the shares of any class or series of stock of a constituent corporation if the holders thereof are required by the terms of an agreement of merger or consolidation pursuant to §§ 251, 252, 254, 255, 256, 257, 258, 263 and 264 of this title to accept for such stock anything except: |
a. | Shares of stock of the corporation surviving or resulting from such merger or consolidation, or depository receipts in respect thereof; |
b. | Shares of stock of any other corporation, or depository receipts in respect thereof, which shares of stock (or depository receipts in respect thereof) or depository receipts at the effective date of the merger or consolidation will be either listed on a national securities exchange or held of record by more than 2,000 holders; |
c. | Cash in lieu of fractional shares or fractional depository receipts described in the foregoing paragraphs (b)(2) a. and b. of this section; or |
d. | Any combination of the shares of stock, depository receipts and cash in lieu of fractional shares or fractional depository receipts described in the foregoing paragraphs (b)(2)a., b. and c. of this section. |
3. | In the event all of the stock of a subsidiary Delaware corporation party to a merger effected under § 253 or § 267 of this title is not owned by the parent immediately prior to the merger, appraisal rights shall be available for the shares of the subsidiary Delaware corporation. |
4. | In the event of an amendment to a corporation’s certificate of incorporation contemplated by § 363(a) of this title, appraisal rights shall be available as contemplated by § 363(b) of this title, and the procedures of this section, including those set forth in subsections (d) and (e) of this |
(c) | Any corporation may provide in its certificate of incorporation that appraisal rights under this section shall be available for the shares of any class or series of its stock as a result of an amendment to its certificate of incorporation, any merger or consolidation in which the corporation is a constituent corporation or the sale of all or substantially all of the assets of the corporation. If the certificate of incorporation contains such a provision, the provisions of this section, including those set forth in subsections (d), (e), and (g) of this section, shall apply as nearly as is practicable. |
(d) | Appraisal rights shall be perfected as follows: |
1. | If a proposed merger or consolidation for which appraisal rights are provided under this section is to be submitted for approval at a meeting of stockholders, the corporation, not less than 20 days prior to the meeting, shall notify each of its stockholders who was such on the record date for notice of such meeting (or such members who received notice in accordance with § 255(c) of this title) with respect to shares for which appraisal rights are available pursuant to subsection (b) or (c) of this section that appraisal rights are available for any or all of the shares of the constituent corporations, and shall include in such notice a copy of this section and, if 1 of the constituent corporations is a nonstock corporation, a copy of § 114 of this title. Each stockholder electing to demand the appraisal of such stockholder’s shares shall deliver to the corporation, before the taking of the vote on the merger or consolidation, a written demand for appraisal of such stockholder’s shares; provided that a demand may be delivered to the corporation by electronic transmission if directed to an information processing system (if any) expressly designated for that purpose in such notice. Such demand will be sufficient if it reasonably informs the corporation of the identity of the stockholder and that the stockholder intends thereby to demand the appraisal of such stockholder’s shares. A proxy or vote against the merger or consolidation shall not constitute such a demand. A stockholder electing to take such action must do so by a separate written demand as herein provided. Within 10 days after the effective date of such merger or consolidation, the surviving or resulting corporation shall notify each stockholder of each constituent corporation who has complied with this subsection and has not voted in favor of or consented to the merger or consolidation of the date that the merger or consolidation has become effective; or |
2. | If the merger or consolidation was approved pursuant to § 228, § 251(h), § 253, or § 267 of this title, then either a constituent corporation before the effective date of the merger or consolidation or the surviving or resulting corporation within 10 days thereafter shall notify each of the holders of any class or series of stock of such constituent corporation who are entitled to appraisal rights of the approval of the merger or consolidation and that appraisal rights are available for any or all shares of such class or series of stock of such constituent corporation, and shall include in such notice a copy of this section and, if 1 of the constituent corporations is a nonstock corporation, a copy of § 114 of this title. Such notice may, and, if given on or after the effective date of the merger or consolidation, shall, also notify such stockholders of the effective date of the merger or consolidation. Any stockholder entitled to appraisal rights may, within 20 days after the date of giving such notice or, in the case of a merger approved pursuant to § 251(h) of this title, within the later of the consummation of the offer contemplated by § 251(h) of this title and 20 days after the date of giving such notice, demand in writing from the surviving or resulting corporation the appraisal of such holder’s shares; provided that a demand may be delivered to the corporation by electronic transmission if directed to an information processing system (if any) expressly designated for that purpose in such notice. Such demand will be sufficient if it reasonably informs the corporation of the identity of the stockholder and that the stockholder intends thereby to demand the appraisal of such holder’s shares. If such notice did not notify stockholders of the effective date of the merger or consolidation, either (i) each such constituent corporation shall send a second notice before the effective date of the merger or consolidation notifying each of the holders of any class or series of stock of such constituent corporation that are entitled to appraisal rights of the effective date of the merger or consolidation or (ii) the surviving or resulting |
(e) | Within 120 days after the effective date of the merger or consolidation, the surviving or resulting corporation or any stockholder who has complied with subsections (a) and (d) of this section hereof and who is otherwise entitled to appraisal rights, may commence an appraisal proceeding by filing a petition in the Court of Chancery demanding a determination of the value of the stock of all such stockholders. Notwithstanding the foregoing, at any time within 60 days after the effective date of the merger or consolidation, any stockholder who has not commenced an appraisal proceeding or joined that proceeding as a named party shall have the right to withdraw such stockholder’s demand for appraisal and to accept the terms offered upon the merger or consolidation. Within 120 days after the effective date of the merger or consolidation, any stockholder who has complied with the requirements of subsections (a) and (d) of this section hereof, upon request given in writing (or by electronic transmission directed to an information processing system (if any) expressly designated for that purpose in the notice of appraisal), shall be entitled to receive from the corporation surviving the merger or resulting from the consolidation a statement setting forth the aggregate number of shares not voted in favor of the merger or consolidation (or, in the case of a merger approved pursuant to § 251(h) of this title, the aggregate number of shares (other than any excluded stock (as defined in § 251(h)(6)d. of this title)) that were the subject of, and were not tendered into, and accepted for purchase or exchange in, the offer referred to in § 251(h)(2)), and, in either case, with respect to which demands for appraisal have been received and the aggregate number of holders of such shares. Such statement shall be given to the stockholder within 10 days after such stockholder’s request for such a statement is received by the surviving or resulting corporation or within 10 days after expiration of the period for delivery of demands for appraisal under subsection (d) of this section hereof, whichever is later. Notwithstanding subsection (a) of this section, a person who is the beneficial owner of shares of such stock held either in a voting trust or by a nominee on behalf of such person may, in such person’s own name, file a petition or request from the corporation the statement described in this subsection. |
(f) | Upon the filing of any such petition by a stockholder, service of a copy thereof shall be made upon the surviving or resulting corporation, which shall within 20 days after such service file in the office of the Register in Chancery in which the petition was filed a duly verified list containing the names and addresses of all stockholders who have demanded payment for their shares and with whom agreements as to the value of their shares have not been reached by the surviving or resulting corporation. If the petition shall be filed by the surviving or resulting corporation, the petition shall be accompanied by such a duly verified list. The Register in Chancery, if so ordered by the Court, shall give notice of the time and place fixed for the hearing of such petition by registered or certified mail to the surviving or resulting corporation and to the stockholders shown on the list at the addresses therein stated. Such notice shall also be given by 1 or more publications at least 1 week before the day of the hearing, in a newspaper of general circulation published in the City of Wilmington, Delaware or such publication as the Court deems advisable. The forms of the notices by mail and by publication shall be approved by the Court, and the costs thereof shall be borne by the surviving or resulting corporation. |
(g) | At the hearing on such petition, the Court shall determine the stockholders who have complied with this section and who have become entitled to appraisal rights. The Court may require the stockholders |
(h) | After the Court determines the stockholders entitled to an appraisal, the appraisal proceeding shall be conducted in accordance with the rules of the Court of Chancery, including any rules specifically governing appraisal proceedings. Through such proceeding the Court shall determine the fair value of the shares exclusive of any element of value arising from the accomplishment or expectation of the merger or consolidation, together with interest, if any, to be paid upon the amount determined to be the fair value. In determining such fair value, the Court shall take into account all relevant factors. Unless the Court in its discretion determines otherwise for good cause shown, and except as provided in this subsection, interest from the effective date of the merger through the date of payment of the judgment shall be compounded quarterly and shall accrue at 5% over the Federal Reserve discount rate (including any surcharge) as established from time to time during the period between the effective date of the merger and the date of payment of the judgment. At any time before the entry of judgment in the proceedings, the surviving corporation may pay to each stockholder entitled to appraisal an amount in cash, in which case interest shall accrue thereafter as provided herein only upon the sum of (1) the difference, if any, between the amount so paid and the fair value of the shares as determined by the Court, and (2) interest theretofore accrued, unless paid at that time. Upon application by the surviving or resulting corporation or by any stockholder entitled to participate in the appraisal proceeding, the Court may, in its discretion, proceed to trial upon the appraisal prior to the final determination of the stockholders entitled to an appraisal. Any stockholder whose name appears on the list filed by the surviving or resulting corporation pursuant to subsection (f) of this section and who has submitted such stockholder’s certificates of stock to the Register in Chancery, if such is required, may participate fully in all proceedings until it is finally determined that such stockholder is not entitled to appraisal rights under this section. |
(i) | The Court shall direct the payment of the fair value of the shares, together with interest, if any, by the surviving or resulting corporation to the stockholders entitled thereto. Payment shall be so made to each such stockholder, in the case of holders of uncertificated stock forthwith, and the case of holders of shares represented by certificates upon the surrender to the corporation of the certificates representing such stock. The Court’s decree may be enforced as other decrees in the Court of Chancery may be enforced, whether such surviving or resulting corporation be a corporation of this State or of any state. |
(j) | The costs of the proceeding may be determined by the Court and taxed upon the parties as the Court deems equitable in the circumstances. Upon application of a stockholder, the Court may order all or a portion of the expenses incurred by any stockholder in connection with the appraisal proceeding, including, without limitation, reasonable attorney’s fees and the fees and expenses of experts, to be charged pro rata against the value of all the shares entitled to an appraisal. |
(k) | From and after the effective date of the merger or consolidation, no stockholder who has demanded appraisal rights as provided in subsection (d) of this section shall be entitled to vote such stock for any purpose or to receive payment of dividends or other distributions on the stock (except dividends or other distributions payable to stockholders of record at a date which is prior to the effective date of the merger or consolidation); provided, however, that if no petition for an appraisal shall be filed within the time provided in subsection (e) of this section, or if such stockholder shall deliver to the surviving or resulting corporation a written withdrawal of such stockholder’s demand for an appraisal and an acceptance of the merger or consolidation, either within 60 days after the effective date of the merger or consolidation as provided in subsection (e) of this section or thereafter with the written approval of |
(l) | The shares of the surviving or resulting corporation to which the shares of such objecting stockholders would have been converted had they assented to the merger or consolidation shall have the status of authorized and unissued shares of the surviving or resulting corporation. |
| | | If to the Rights Agent, to it at: | ||||
| | | | | |||
| | | Olde Monmouth Stock Transfer Co., Inc. | ||||
| | | Telephone: | | | (732) 872-2727, Ext. 101 | |
| | | Email: | | | ||
| | | Attention: | | | Matthew J. Troster, President | |
| | | | | |||
| | | If to Parent, to it at: | ||||
| | | | | |||
| | | Rexahn Pharmaceuticals, Inc. | ||||
| | | Telephone: | | | [•] | |
| | | Email: | | | [•] | |
| | | Attention: | | | [•] | |
| | | | | |||
| | | with a copy to: | ||||
| | | | | |||
| | | [•] | ||||
| | | Telephone: | | | [•] | |
| | | Email: | | | [•] | |
| | | Attention: | | | [•] | |
| | | | | |||
| | | If to the CVR Representative, to it at: | ||||
| | | | | |||
| | | Shareholder Representative Services LLC | ||||
| | | 950 17th Street, Suite 1400 | ||||
| | | Denver, CO 80202 | ||||
| | | Telephone: | | | (303) 648-4085 | |
| | | Email: | | | ||
| | | Attention: | | | Managing Director | |
| | | | | |||
| | | with a copy to: | ||||
| | | | | |||
| | | Hogan Lovells US LLP | ||||
| | | 100 International Drive, Suite 2000 | ||||
| | | Baltimore, MD 21202 | ||||
| | | Attention: Asher M. Rubin; William I. Intner | ||||
| | | Email: [email protected]; [email protected] | ||||
| | | REXAHN PHARMACEUTICALS, INC. | ||||
| | | | | |||
| | | By: | | | ||
| | | Name: | | | ||
| | | Title: | | | ||
| | | | | |||
| | | OLDE MONMOUTH STOCK TRANSFER CO., INC. | ||||
| | | | | |||
| | | By: | | | ||
| | | Name: | | | ||
| | | Title: | | | ||
| | | | | |||
| | | SHAREHOLDER REPRESENTATIVE SERVICES LLC, solely in its capacity as the CVR Representative | ||||
| | | | | |||
| | | By: | | | ||
| | | Name: | | | ||
| | | Title: | | | ||
(a) | The undersigned registrant hereby undertakes: |
(1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
(i) | To include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
(ii) | To reflect in the prospectus any facts or events arising after the effective date of this registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in this registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed |
(iii) | To include any material information with respect to the plan of distribution not previously disclosed in this registration statement or any material change to such information in this registration statement. |
(2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
(3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
(4) | That, for the purpose of determining liability under the Securities Act of 1933, to any purchaser: if the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness; provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
(5) | That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
(i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
(ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
(iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
(iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
(b) | The undersigned registrant hereby undertakes as follows: that prior to any public reoffering of the securities registered hereunder through use of a prospectus which is a part of this registration statement, by any person or party who is deemed to be an underwriter within the meaning of Rule 145(c), the issuer undertakes that such reoffering prospectus will contain the information called for by the applicable registration form with respect to reofferings by persons who may be deemed underwriters, in addition to the information called for by the other items of the applicable form. |
(c) | The undersigned registrant hereby undertakes as follows: that every prospectus (i) that is filed pursuant to paragraph (b) immediately preceding, or (ii) that purports to meet the requirements of Section 10(a)(3) of the Securities Act of 1933 and is used in connection with an offering of securities |
(d) | The undersigned registrant hereby undertakes to respond to requests for information that is incorporated by reference into this proxy statement/prospectus/information statement pursuant to Item 4, 10(b), 11, or 13 of Form S-4, within one business day of receipt of such request, and to send the incorporated documents by first class mail or other equally prompt means. This includes information contained in documents filed subsequent to the effective date of the registration statement through the date of responding to the request. |
(e) | The undersigned registrant hereby undertakes to supply by means of a post-effective amendment all information concerning a transaction, and the company being acquired involved therein, that was not the subject of and included in the registration statement when it became effective. |
(f) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue. |
EXHIBIT NUMBER | | | DESCRIPTION OF DOCUMENT |
| | | Agreement and Plan of Merger, dated as of June 17, 2020, by and among the Registrant, Razor Merger Sub, Inc. and Ocuphire Pharma, Inc. (included as Annex A to the proxy statement/prospectus/information statement forming a part of this Registration Statement). | |
| | | Form of CVR Agreement by and among the Registrant, Shareholder Representative Services LLC, as Representative, and Olde Monmouth Stock Transfer Co., Inc., as Rights Agent (included in Annex G to the proxy statement/prospectus/information statement forming a part of this Registration Statement). | |
| | | Form of Ocuphire Voting Agreement, by and between the Registrant, Ocuphire Pharma, Inc. and certain stockholders of Ocuphire (included in Annex A to the proxy statement/prospectus/information statement forming a part of this Registration Statement). | |
| | | Form of Lock-Up Agreement, by and between the Registrant, Ocuphire Pharma, Inc. and certain stockholders of the Registrant and Ocuphire (included in Annex A to the proxy statement/prospectus/information statement forming a part of this Registration Statement). | |
| | | First Amendment to Agreement and Plan of Merger and Reorganization, dated as of June 29, 2020, by and among Rexahn, Merger Sub and Ocuphire (included in Annex A to the proxy statement/prospectus/information statement forming a part of this Registration Statement). | |
| | | Amended and Restated Certificate of Incorporation of the Registrant (incorporated by reference to Appendix G to the Registrant’s Definitive Proxy Statement on Schedule 14A, filed on April 29, 2005). | |
| | | Certificate of Amendment of Amended and Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K, filed on May 5, 2017). | |
| | | Certificate of Amendment of Amended and Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K, filed on August 30, 2018). | |
| | | Certificate of Amendment of Amended and Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K, filed on April 12, 2019). | |
| | | Amended and Restated Bylaws, as amended, of the Registrant through March 21, 2014 (incorporated by reference to Exhibit 3.2 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2013). | |
| | | Specimen Certificate for the Registrant’s Common Stock, par value $.0001 per share (incorporated by reference to Exhibit 4.3 to the Registrant’s Registration Statement on Form S-8 (File No. 333-129294), filed on October 28, 2005). | |
| | | Form of Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed on November 6, 2015). | |
| | | Form of Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed on February 26, 2016). | |
| | | Form of Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed on September 14, 2016). | |
| | | Form of Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed on June 7, 2017). | |
| | | Form of Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed on October 13, 2017). | |
| | | Form of Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed on October 19, 2018). | |
| | | Form of Common Stock Purchase Warrant (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed on January 25, 2019). | |
| | | Stockholders Agreement, dated as of April 10, 2018, among Ocuphire Pharma, Inc. and Stockholders as defined therein. |
EXHIBIT NUMBER | | | DESCRIPTION OF DOCUMENT |
| | | Form of Series A/B Warrants (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, filed on July 1, 2020). | |
| | | Opinion of Hogan Lovells US LLP. | |
| | | Legal Opinion of Honigman LLP regarding tax matters. | |
| | | Legal Opinion of Hogan Lovells US LLP regarding tax matters. | |
| | | Rexahn Pharmaceuticals, Inc. Stock Option Plan, as amended (incorporated by reference to Exhibit 4.4 to the Registrant’s Registration Statement on Form S-8 (File No. 333-129294), filed on October 28, 2005). | |
| | | Form of Stock Option Grant Agreement for Employees (incorporated by reference to Exhibit 4.5.1 to the Registrant’s Registration Statement on Form S-8 (File No. 333-129294), filed on October 28, 2005). | |
| | | Form of Stock Option Grant Agreement for Non-Employee Directors and Consultants (incorporated by reference to Exhibit 4.5.2 to the Registrant’s Registration Statement on Form S-8 (File No. 333-129294), filed on October 28, 2005). | |
| | | Rexahn Pharmaceuticals, Inc. 2013 Stock Option Plan, as amended and restated (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on June 10, 2016) | |
| | | First Amendment to the Rexahn Pharmaceuticals, Inc. 2013 Stock Option Plan, as amended and restated as of June 9, 2016 (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on April 13, 2017). | |
| | | Form of Stock Option Grant Agreement under the Rexahn Pharmaceuticals, Inc. 2013 Stock Option Plan (incorporated by reference to Exhibit 10.5 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2015). | |
| | | Form of Restricted Stock Unit Agreement under the Rexahn Pharmaceuticals, Inc. 2013 Stock Option Plan (incorporated by reference to Exhibit 10.7 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2018). | |
| | | Employment Agreement, dated as of January 2, 2018, by and between Rexahn Pharmaceuticals, Inc. and Douglas Swirsky (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on January 4, 2018). | |
| | | Amendment to Employment Agreement, dated as of November 14, 2018, by and between Rexahn Pharmaceuticals, Inc. and Douglas Swirsky (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on November 16, 2018). | |
| | | Lease Agreement, dated June 5, 2009, by and between Rexahn Pharmaceuticals, Inc. and The Realty Associates Fund V, L.P. (incorporated by reference to Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2009). | |
| | | First Amendment to Lease Agreement, dated as of June 7, 2013, by and between Rexahn Pharmaceuticals, Inc. and SG Plaza Holdings, LLC (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2013). | |
| | | Second Amendment to Lease Agreement, dated as of July 26, 2014, by and between Rexahn Pharmaceuticals, Inc. and SG Plaza Holdings, LLC (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2014). | |
| | | Third Amendment to Lease Agreement, dated as of May 6, 2015, by and between Rexahn Pharmaceuticals, Inc. and SG Plaza Holdings, LLC (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2015). | |
| | | Fourth Amendment to Lease Agreement, dated as of April 4, 2016, by and between Rexahn Pharmaceuticals, Inc. and SG Plaza Holdings, LLC (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2016). |
EXHIBIT NUMBER | | | DESCRIPTION OF DOCUMENT |
| | | Fifth Amendment to Lease Agreement, dated as of April 13, 2017, by and between Rexahn Pharmaceuticals, Inc. and SG Plaza Holdings, LLC (incorporated by reference to Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2017). | |
| | | Sixth Amendment to Lease Agreement, dated as of March 19, 2019, by and between Rexahn Pharmaceuticals, Inc. and SG Plaza Holdings, LLC (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2019). | |
| | | Form of Securities Purchase Agreement, dated as of November 6, 2015, by and between Rexahn Pharmaceuticals, Inc. and the purchasers identified on the signature pages thereto (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on November 6, 2015). | |
| | | Form of Securities Purchase Agreement, dated as of February 26, 2016, by and between Rexahn Pharmaceuticals, Inc. and the purchasers identified on the signature pages thereto (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on February 26, 2016). | |
| | | Form of Securities Purchase Agreement, dated as of September 14, 2016, by and between Rexahn Pharmaceuticals, Inc. and the purchasers identified on the signature pages thereto (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on September 14, 2016). | |
| | | Form of Securities Purchase Agreement, dated as of June 6, 2017, by and between Rexahn Pharmaceuticals, Inc. and the purchasers identified on the signature pages thereto (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on June 7, 2017). | |
| | | Form of Securities Purchase Agreement, dated as of October 13, 2017, by and between Rexahn Pharmaceuticals, Inc. and the purchasers identified on the signature pages thereto (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on October 13, 2017). | |
| | | Form of Securities Purchase Agreement, dated as of October 17, 2018, by and between Rexahn Pharmaceuticals, Inc. and the purchasers identified on the signature pages thereto (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on October 19, 2018). | |
| | | Collaboration and License Agreement, dated as of February 25, 2019, between BioSense Global, LLC and Rexahn Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2019). | |
| | | Amendment No. 1 to Collaboration and License Agreement, dated as of August 24, 2019, between BioSense Global LLC and Rexahn Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on August 29, 2019). | |
| | | Amendment No. 2 to Collaboration and License Agreement, dated as of March 10, 2020 between BioSense Global LLC and Rexahn Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q filed on May 7, 2020). | |
| | | Employment Agreement, dated as of October 1, 2018, between Ocuphire Pharma, Inc. and Mina Sooch. | |
| | | Amended and Restated Employment Agreement by and among Ocuphire Pharma, Inc. and Mina Sooch, to be effective as of the Closing. | |
| | | Employment Agreement, dated as of October 1, 2018, between Ocuphire Pharma, Inc. and Bernhard Hoffmann. | |
| | | Amended and Restated Employment Agreement by and among Ocuphire Pharma, Inc. and Bernhard Hoffmann, to be effective as of the Closing. | |
| | | Form of Indemnification Agreement. |
EXHIBIT NUMBER | | | DESCRIPTION OF DOCUMENT |
| | | Sublicense Agreement, dated as of January 21, 2020, by and between Ocuphire Pharma, Inc. and Apexian Pharmaceuticals, Inc. | |
| | | First Amendment to Sublicense Agreement, dated as of June 4, 2020, by and between Apexian Pharmaceuticals, Inc. and Ocuphire Pharma, Inc. | |
| | | Lease Agreement, dated as of May 19, 2019, by and between Ocuphire Pharma, Inc. and Duke & Duke, LP. | |
| | | First Amendment to Lease Agreement, dated as of October 29, 2019, by and between Ocuphire Pharma, Inc. and Duke & Duke, LP. | |
| | | Ocuphire Pharma, Inc. 2018 Equity Incentive Plan, dated as of April 9, 2019. | |
| | | First Amendment to 2018 Equity Incentive Plan, dated as of December 23, 2019. | |
| | | Form of Option Agreement issuable under the Ocuphire Pharma, Inc. 2018 Equity Incentive Plan. | |
| | | Ocuphire Pharma, Inc. 2020 Omnibus Equity Incentive Plan (included as Annex D to the proxy statement/prospectus/information statement forming a part of this Registration Statement). | |
| | | Amended and Restated Mezz Note Purchase Agreement, dated as of January 22, 2019, by and among Ocuphire Pharma, Inc. and each of the purchasers set forth therein. | |
| | | First Amendment to Amended and Restated Mezz Note Purchase Agreement, dated as of November 20, 2019, by and among Ocuphire Pharma, Inc. and each of the purchasers party to the Amended and Restated Mezz Note Purchase Agreement. | |
| | | Note Conversion Agreement, dated as of June 8, 2020, by and among Ocuphire Pharma, Inc. and each of the purchasers party to the Amended and Restated Mezz Note Purchase Agreement. | |
| | | Amended and Restated Securities Purchase Agreement, dated as of June 29, 2020, by and among Rexahn, Ocuphire and the investors party thereto (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on July 1, 2020). | |
| | | Form of Financing Lock-Up Agreement, by and among Rexahn, Ocuphire, and the investors party thereto (incorporated by reference to Exhibit 10.2 to the Registrant’s Current Report on Form 8-K, filed on July 1, 2020). | |
| | | Form of Leak-Out Agreement, by and between Rexahn and the investors party thereto (incorporated by reference to Exhibit 10.3 to the Registrant’s Current Report on Form 8-K, filed on July 1, 2020). | |
| | | Exclusive License Agreement, dated as of February 8, 2020, by and between Rexahn and Zhejiang HaiChang Biotechnology Co., Ltd. | |
| | | Warrant Exchange Agreement, dated July 31, 2020, by and between Rexahn Pharmaceuticals, Inc. and Armistice Capital Master Fund Ltd. (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on August 3, 2020). | |
| | | Warrant Exchange Agreement, dated September 1, 2020, by and between Rexahn Pharmaceuticals, Inc. and Anson Investments Master Fund LP (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on September 2, 2020). | |
| | | Warrant Exchange Agreement, dated September 10, 2020, by and between Rexahn Pharmaceuticals, Inc. and Empery Asset Master, Ltd. (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed on September 11, 2020). | |
| | | Warrant Exchange Agreement, dated September 10, 2020, by and between Rexahn Pharmaceuticals, Inc. and Empery Tax Efficient, LP (incorporated by reference to Exhibit 10.2 to the Registrant’s Current Report on Form 8-K, filed on September 11, 2020). | |
| | | Warrant Exchange Agreement, dated September 10, 2020, by and between Rexahn Pharmaceuticals, Inc. and Empery Tax Efficient II, LP (incorporated by reference to Exhibit 10.3 to the Registrant’s Current Report on Form 8-K, filed on September 11, 2020). | |
| | | Subsidiaries of the Registrant | |
| | | Consent of Baker Tilly US, LLP (formerly known as Baker Tilly Virchow Krause, LLP). |
EXHIBIT NUMBER | | | DESCRIPTION OF DOCUMENT |
| | | Consent of Hogan Lovells US LLP (contained in Exhibit 5.1). | |
| | | Consent of Honigman LLP (contained in Exhibit 8.1). | |
| | | Consent of Hogan Lovells US LLP (contained in Exhibit 8.2). | |
| | | Consent of Ernst & Young, LLP. | |
| | | Power of Attorney. | |
| | | Form of Proxy Card for Special Meeting of Stockholders of Registrant. | |
| | | Proposed Amendment to the Amended and Restated Certificate of Incorporation of Registrant for the Rexahn Reverse Stock Split (included as Annex B to the proxy statement/prospectus/information statement forming a part of this Registration Statement). | |
| | | Proposed Amendment to the Amended and Restated Certificate of Incorporation of Registrant for the Rexahn Name Change (included as Annex C to the proxy statement/prospectus/information statement forming a part of this Registration Statement). | |
| | | Opinion of Oppenheimer & Co. Inc., financial advisor to Rexahn (included as Annex E to the proxy statement/prospectus/information statement forming part of this Registration Statement). | |
| | | Consent of Oppenheimer & Co. Inc., financial advisor to Rexahn. | |
| | | Consent of Mina Sooch to be named as a Director. | |
| | | Consent of Sean Ainsworth to be named as a Director. | |
| | | Consent of Alan R. Meyer to be named as a Director. | |
| | | Consent of James S. Manuso to be named as a Director. | |
| | | Consent of Cam Gallagher to be named as a Director. | |
| | | Consent of Susan K. Benton to be named as a Director. | |
101.INS# | | | XBRL Instance Document |
101.SCH# | | | XBRL Taxonomy Extension Schema Document |
101.CAL# | | | XBRL Taxonomy Extension Calculation Linkbase Document |
101.DEF# | | | XBRL Taxonomy Extension Definition Linkbase Document |
101.LAB# | | | XBRL Taxonomy Extension Label Linkbase Document |
101.PRE# | | | XBRL Taxonomy Extension Presentation Linkbase Document |
* | Indicates management contract or compensatory plan. |
+ | Certain schedules and exhibits have been omitted pursuant to Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule and/or exhibit will be furnished to the SEC upon request. |
++ | Portions of this exhibit have been omitted in compliance with Item 601 of Regulation S-K. |
# | Previously filed. |
| | | REXAHN PHARMACEUTICALS, INC. | ||||
| | | | | |||
| | | By: | | | /s/ Douglas J. Swirsky | |
| | | Name: | | | Douglas J. Swirsky | |
| | | Title: | | | President and Chief Executive Officer | |
Signature | | | Title | | | Date |
/s/ Douglas J. Swirsky | | | President, Chief Executive Officer and Director (Principal Executive, Financial and Accounting Officer) | | | September 30, 2020 |
Douglas J. Swirsky | | |||||
| | | | | |||
* | | | Chairman | | | September 30, 2020 |
Peter Brandt | | |||||
| | | | | |||
* | | | Director | | | September 30, 2020 |
Charles Beever | | |||||
| | | | | |||
* | | | Director | | | September 30, 2020 |
Kwang Soo Cheong | | |||||
| | | | | |||
* | | | Director | | | September 30, 2020 |
Ben Gil Price | | |||||
| | | | | |||
* | | | Director | | | September 30, 2020 |
Richard J. Rodgers | | |||||
| | | | | |||
* | | | Director | | | September 30, 2020 |
Lara Sullivan | |
*By: | | | /s/ Douglas J. Swirsky | | | |
| | | | | |||
| | | Douglas J. Swirsky Attorney-in-fact | | |